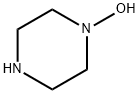
Piperazine, 1-hydroxy- synthesis
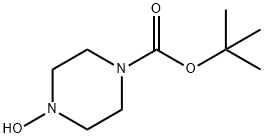
- Product Name:Piperazine, 1-hydroxy-
- CAS Number:69395-49-9
- Molecular formula:C4H10N2O
- Molecular Weight:102.14
Yield:-
Reaction Conditions:
in dichloromethane at 20; for 0.75 h;Inert atmosphere;
Steps:
3',6'-Bis(dimethylamino)-6-(4-hydroxypiperazine-1-carbonyl)-3H-spiro[isobenzofuran-1,9'-xanthen]-3-one (10):
Trifluoroacetic acid (200 μL) was added to a solution of hydroxylamine 54 (20.7 mg, 102 μmol, 1.10equiv) in dichloromethane (800 μL). The resulting solution was stirred at room temperature for 45 minthen concentrated under reduced pressure. 6-TAMRA (40.0 mg, 92.9 μmol, 1 equiv) was added and themixture was dissolved in N,N-dimethylformamide (1.0 mL). N,N-Diisopropylethylamine (80.9 μL, 465μmol, 5.00 equiv) and 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxidehexafluorophosphate (HATU, 38.9 mg, 102 μmol, 1.10 equiv) were then sequentially added to thesolution. The reaction mixture was stirred at room temperature for 2 h, diluted with water, and purified byautomated C18 reverse phase column chromatography (30 g C18 silica gel, 25 μm spherical particles,eluent: H2O+0.1% TFA (5 CV), gradient 0→100% MeCN/H2O+0.1% TFA (15 CV)) and flash columnchromatography on silica gel (eluent: 70% CMA in chloroform) to provide TAMRA-hydroxylamine 10(26.6 mg, 56%) as a dark violet solid.
References:
Kang, Dahye;Lee, Sanghyeon;Kim, Justin [Chem,2022,vol. 8,# 8,p. 2260 - 2277] Location in patent:supporting information
109142-44-1
2 suppliers
inquiry

69395-49-9
3 suppliers
inquiry
109142-42-9
2 suppliers
inquiry

69395-49-9
3 suppliers
inquiry
110-85-0
548 suppliers
$5.00/5G

69395-49-9
3 suppliers
inquiry