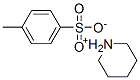
piperidinium toluene-4-sulphonate synthesis
- Product Name:piperidinium toluene-4-sulphonate
- CAS Number:14034-66-3
- Molecular formula:C12H19NO3S
- Molecular Weight:257.3492

110-89-4
3 suppliers
$15.96/100ML
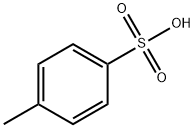
104-15-4
722 suppliers
$133.00/500g
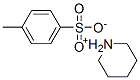
14034-66-3
2 suppliers
inquiry
Yield:14034-66-3 97%
Reaction Conditions:
in acetone at 15 - 20; for 0.5 h;
Steps:
Piperidinium tosylate (1b)2:
0.43 g piperidine (5.0 mmol) and 0.95 g (5.0 mmol) p-toluenesulfonic acid monohydrate were mixed in 15 mL acetone, the mixture was stirred for 30 mins at room temperature (15-20 °C), evaporation of the solvent gave the crude product, which was washed with 30 mL n-hexane to give a pure piperidinium tosylate (1.34 g) as a white crystal, the yield is 97%. m.p.: 132.3-134.2 °C (reference1 m.p. = 128-130 °C); 1H NMR (400MHz, CDCl3): δ 8.62 (br, 2H), 7.76 (d, J = 8.0 Hz, 2H), 7.21(d, J = 8.0 Hz, 2H), 3.13 (t, J = 8.0 Hz, 4H), 2.38 (s, 3H), 1.80-1.77(m, 4H), 1.60-1.59 (m, 2H) ppm; 13C NMR (100 MHz, CDCl3): δ 141.72, 140.47, 129.00, 125.79, 44.79, 22.44, 22.27, 21.34 ppm; IR (KBr): 3450, 2951, 2841, 2513, 1601, 1458, 1394, 1188, 1124, 1036, 1011, 818 cm-1.
References:
Gao, Lan;Liu, Taoping;Tao, Xiaochun;Huang, Yongmin [Tetrahedron Letters,2016,vol. 57,# 44,p. 4905 - 4909] Location in patent:supporting information