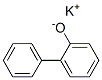
potassium 2-biphenylate synthesis
- Product Name:potassium 2-biphenylate
- CAS Number:13707-65-8
- Molecular formula:C12H9KO
- Molecular Weight:208.2976
Yield:92379-11-8 82%
Reaction Conditions:
in N-methyl-acetamide;toluene;mineral oil;
Steps:
18 EXAMPLE 18
EXAMPLE 18 Following the procedure of Example 2, anhydrous potassium 2-phenylphenate was prepared from 3.223 moles of 2-phenylphenol in toluene. The toluene was removed and replaced by 944.5 grams of dimethylformamide. The solution was cooled to 100° C. and carbon dioxide was passed in for 6 hours, with stirring. At the end of this time, the conversion was 48.6%. The solution was cooled to about 60° C. and 16.1 grams (537 mmol.) of an 80% (by weight) dispersion of sodium hydride in mineral oil was added. The mixture was stirred for 4 hours at 60° C., with two additional portions of sodium hydride being added during the last 2 hours. The resulting thick purple mixture was stirred in a carbon dioxide atmosphere and heated to 100° C. over 4 hours. Heating at 100° C. was continued overnight, after which the conversion was 73.9%. In the next 2 hours, 806 mmol. of additional sodium hydride was added in two portions, after which heating with carbon dioxide at 100° C was continued for 8 hours. The conversion had then reached 79%. About one third of the mixture was added to 2000 ml. of vigorously stirred toluene. The supernatent liquid was removed by decantation and the heavy viscous layer was extracted two more times with 500-ml. portions of toluene, dissolved in 500 ml. of water and acidified with aqueous hydrochloric acid solution. The product which precipitated was washed with water and dried to yield 562.6 grams of the desired 4-hydroxy-3-phenylbenzoic acid (82% conversion).
References:
US4873367,1989,A
90-43-7
321 suppliers
$10.00/5g

13707-65-8
9 suppliers
inquiry
![[1,1'-Biphenyl]-3-carboxylic acid, 6-hydroxy-](/CAS/20200515/GIF/92379-11-8.gif)