
Potassium Acetate synthesis
- Product Name:Potassium Acetate
- CAS Number:127-08-2
- Molecular formula:C2H3KO2
- Molecular Weight:98.14
2 CH3COOH + K2CO3→ 2 CH3CO2K + CO2 + H2O
This sort of reaction is known as an acid-base neutralization reaction. Potassium acetate is the salt that forms along with water as acetic acid and potassium hydroxide are neutralized together.
Conditions/substances to avoid are: moisture, heat, flames, ignition sources, and strong oxidizing agents.
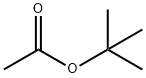
540-88-5
376 suppliers
$22.00/25mL
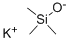
10519-96-7
265 suppliers
$6.00/1g

127-08-2
648 suppliers
$6.00/25g
Yield:127-08-2 77%
Steps:
37 Potassium acetate
EXAMPLE 37 Potassium acetate The procedure of Example 1 was followed using t-butyl acetate (4.0 mL, 30 mmol), potassium trimethylsilanolate (3.81 g, 30 mmol), dry ether (100 mL), and a 3 h reaction time. Potassium acetate (2.25 g, 77% yield) was isolated as a white solid: 1 H NMR (D2 O) δ 1.8 ppm (s, CH3, 3H).
References:
US4723016,1988,A

64-17-5
746 suppliers
$10.00/50g

127-08-2
648 suppliers
$6.00/25g
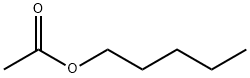
628-63-7
397 suppliers
$19.00/25mL

127-08-2
648 suppliers
$6.00/25g

75-07-0
424 suppliers
$14.00/5mL

127-08-2
648 suppliers
$6.00/25g
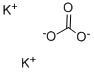
584-08-7
1222 suppliers
$5.00/100g

127-08-2
648 suppliers
$6.00/25g