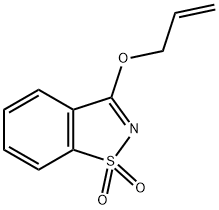
Probenazole synthesis
- Product Name:Probenazole
- CAS Number:27605-76-1
- Molecular formula:C10H9NO3S
- Molecular Weight:223.25

107-18-6
2 suppliers
$24.60/100ml
![3-Chloro-benzo[d]isothiazole 1,1-dioxide](/CAS/GIF/567-19-1.gif)
567-19-1
60 suppliers
$65.00/50mg

27605-76-1
132 suppliers
$65.00/100 mg
Yield:27605-76-1 96%
Reaction Conditions:
Stage #1:allyl alcohol with potassium hydroxide in cyclohexaneReflux;
Stage #2:3-chloro-1,2-benzisothiazole 1,1-dioxide in chlorobenzene at 20 - 30; for 2 h;Reagent/catalyst;Solvent;
Steps:
1.2-1.3; 2.2-2.3
2. Preparation of potassium allyl alcohol:Add 160g of powdered KOH [1eq] to a 1000mL three-necked bottle,100mL of cyclohexane and 300g of allyl alcohol [1.5eq], heated to reflux dehydration, until 50g of water was removed, and the water-containing cyclohexane was distilled off. An allyl alcohol solution of potassium allyl alcohol was obtained.3. Preparation of probenazole by substitution:Add the above allyl alcohol solution of potassium allyl alcohol dropwise to the chlorobenzene solution of chloride,Control the reaction at 20-30 degrees for 2 hours. Remove potassium chloride by filtration, and then control the temperature not to exceed 100 degrees,The excess allyl alcohol was distilled off. The residue was cooled to 0 degrees to obtain 585 g of probenazole, a yield of 96%.
References:
Tianjin North Food Co., Ltd.;Li Shengbin;Liu Hongjun;Liu Yongsheng;Cao Shijia;Chang Hong;Wang Yue CN110698424, 2020, A Location in patent:Paragraph 0031; 0037-0041; 0047-0050

107-18-6
2 suppliers
$24.60/100ml
81-07-2
452 suppliers
$13.98/25G

27605-76-1
132 suppliers
$65.00/100 mg
41335-56-2
1 suppliers
inquiry