
Propanamide, N-hydroxy-3-phenoxy- synthesis
- Product Name:Propanamide, N-hydroxy-3-phenoxy-
- CAS Number:1016863-79-8
- Molecular formula:C9H11NO3
- Molecular Weight:181.19

7497-89-4
21 suppliers
$28.60/250mg

1016863-79-8
6 suppliers
inquiry
Yield:1016863-79-8 60%
Reaction Conditions:
with hydroxylamine hydrochloride;sodium methylate in methanol; for 8 h;Reflux;
Steps:
2.2 General procedure A for the preparationof hydroxamic acids
General procedure: 2.2a N-hydroxy-2-phenoxyacetamide (7a): To asolution of NaOMe (1.95 g, 36.11 mmol) in methanol(10 mL) was added hydroxylamine hydrochloride (1.67 g,24.0 mmol) dissolved in methanol (20 mL). The reactionmixture was stirred for 10 min at room temperature and to itwas added methylphenoxyacetate (1.0 g, 6.0 mmol) andrefluxed for 8 h. After completion of the reaction (by TLC),the mixture was cooled to room temperature and acidifiedwith 10% HCl to pH 4.5 to 5.0. The acidified reactionmixture was filtered to remove the salts. Methanol wasevaporated and the residue diluted with water and extractedwith CH2Cl2. The extract was dried over anhydrous Na2SO4and the solvent was evaporated. The resultant crude productwas purified by column chromatography to furnish therequired product 7a (0.62g, 62%) as a white solid.
References:
Dittakavi, Ramachandran;Madhavarao, Nagarajan;Mannam, Krishnamurthy;Nagalingam, Viswanath;Sreenivasulu, Reddymasu [Journal of Chemical Sciences,2020,vol. 132,# 1]
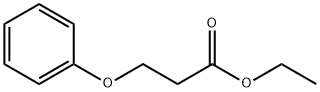
22409-91-2
61 suppliers
$59.21/10g

1016863-79-8
6 suppliers
inquiry

108-95-2
723 suppliers
$14.00/25g

1016863-79-8
6 suppliers
inquiry