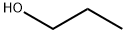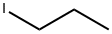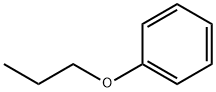
Propoxybenzene synthesis
- Product Name:Propoxybenzene
- CAS Number:622-85-5
- Molecular formula:C9H12O
- Molecular Weight:136.19

106-94-5
564 suppliers
$10.00/5 mL

108-95-2
735 suppliers
$14.00/25g

622-85-5
98 suppliers
$20.00/1g
Yield: 72%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 70; for 5 h;
Steps:
6.1.5. [(4-{[{4-[(Benzylmethanesulfonylamino)methyl]phenyl}(4-ethoxybenzenesulfonyl)amino]methyl}phenyl)methanesulfonylamino]acetic acid methyl ester (P7)
General procedure: The phenol (1.88 g, 20 mmol) was dissolved in a mixture ofDMF (50 mL) and potassium carbonate (4.14 g, 30 mmol), Thenthe bromoethane (2.3 mL, 30 mmol) was added and the mixturewas stirred for about 5 h with heating and reflux at 70 C. Thesolids were removed by filtration and washed with ethyl acetate(140 mL). The filtrate was washed with a saturated brine solutionand dried over anhydrous Na2SO4, and the organic phase was concentratedunder vacuum to afford 1.72 g (70%) of 11b as a colorlessoil. According to the same procedure described for 12a, 11b (1.47 g,12 mmol) was treated with chlorosulfonic acid (2.10 g, 18 mmol)to afford 1.41 g (53%) of 12b as a colorless oil. According to thesame procedure described for 13a, 10a (203 mg, 0.7 mmol) wastreated with 12b (168 mg, 0.76 mmol) to afford 236 mg (71%) of13c as a red oil. According to the same procedure described forP6, 13c (190 mg, 0.40 mmol) was treated with 16a (155 mg,0.46 mmol) to afford 170 mg (58%) of P7 as a white solid.
References:
Liu, Peihong;Du, Yongli;Song, Lianhua;Shen, Jingkang;Li, Qunyi [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 21,p. 7079 - 7088]
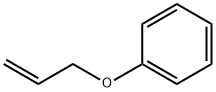
1746-13-0
232 suppliers
$9.00/5g

622-85-5
98 suppliers
$20.00/1g
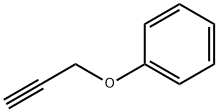
13610-02-1
108 suppliers
$12.00/1g

622-85-5
98 suppliers
$20.00/1g

108-95-2
735 suppliers
$14.00/25g