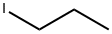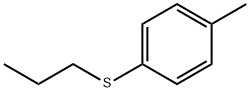
Propyl(4-methylphenyl) sulfide synthesis
- Product Name:Propyl(4-methylphenyl) sulfide
- CAS Number:24599-52-8
- Molecular formula:C10H14S
- Molecular Weight:166.28
Yield:-
Reaction Conditions:
with sodium hydroxide in water at 0; for 1 h;
Steps:
36
A cooled (0 0C) solution of 4-methylthiophenol (Aldrich; 20.0 g; 161 mmol) in MeOH (400 ml) was treated with a 5 N solution of NaOH in water (40 ml.) and with 1-iodopropane (18.0 ml; 185 mmol). The reaction was stirred at at 0 0C for 1 h then concentrated under reduced pressure. The concentrated solution was diluted with EtOAc then washed with brine. The organic phase was dried on MgSO4, filtered and concentrated under reduced pressure to give a residue, which was dissolved in DCM (200 ml) and cooled to at 0 0C. This solution was treated over 20 min with a suspension of 3-chloroperbenzoic acid (83.12 g; 337.2 mmol) in DCM (600 ml). The reaction suspension was stirred at 0 0C for 3 h then treated with a further portion of 3-chloroperbenzoic acid (18.86 g; 76.52 mmol) in DCM (150 ml). The reaction was warmed to RT and stirred for 16 h. The reaction solution was filtered and the filtrate reduced in volume under reduced pressure and diluted with EtOAc, then washed twice with a 1 N solution of NaOH in water and then brine. The organic phase was dried on MgSO4, filtered and concentrated under reduced pressure to give the title compound (24.80 g, 78%) as an oil which solidified upon standing1H NMR (300MHz, DMSO-d6) δ [ppm] 7.76 (d, J= 8.1 Hz, 2H), 7.45 (d, J= 8.1 Hz, 2H), 3.31-3.15 (m, 2H), 2.41 (s, 3H), 1.63-1.42 (m, 2H), 0.89 (t, J= 7.4 Hz, 3H). HPLC (Condition A) Purity 95.4%; Rt 1.4 min.
References:
WO2010/92043,2010,A1 Location in patent:Page/Page column 75
26891-63-4
5 suppliers
inquiry
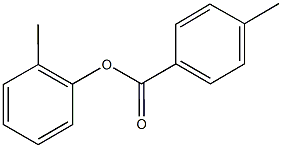
23597-29-7
1 suppliers
inquiry
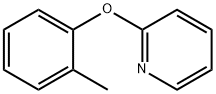
4783-78-2
1 suppliers
inquiry

24599-52-8
8 suppliers
$50.00/1g
![1-Propanol, 3-[(4-methylphenyl)thio]-](/CAS/20180601/GIF/3147-28-2.gif)
3147-28-2
0 suppliers
inquiry
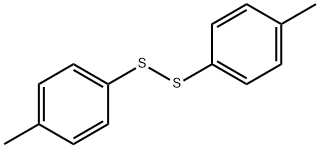
103-19-5
152 suppliers
$19.00/5g

24599-52-8
8 suppliers
$50.00/1g
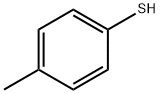
106-45-6
213 suppliers
$10.00/1g
75052-54-9
1 suppliers
inquiry

23597-29-7
1 suppliers
inquiry

24599-52-8
8 suppliers
$50.00/1g
88639-91-2
0 suppliers
inquiry