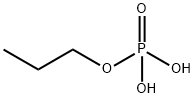
propyl dihydrogen phosphate synthesis
- Product Name:propyl dihydrogen phosphate
- CAS Number:1623-06-9
- Molecular formula:C3H9O4P
- Molecular Weight:140.07
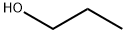
71-23-8
749 suppliers
$12.00/100g

1623-06-9
3 suppliers
inquiry
Yield:1623-06-9 98%
Reaction Conditions:
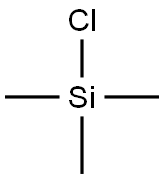
with PPA in water;
Steps:
5 1-Propyldihydrogenphosphate, 3-(perfluoro-C6-18 alkyl-2-propenyl)oxy)-2,2-bis-((perfluoro-C 6-18 alkyl-2-propenyl)oxy)methyl)-
Example 5 1-Propyldihydrogenphosphate, 3-(perfluoro-C6-18 alkyl-2-propenyl)oxy)-2,2-bis-((perfluoro-C 6-18 alkyl-2-propenyl)oxy)methyl)- Into a 500 ml three neck round-bottomed flask are placed 100 g (0.061 mol) of 1-propanol, 3-(perfluoro-C6-18 alkyl-2-propenyl)oxy)-2,2-bis((perfluoro-C6-18 alkyl-2-propenyl)(oxy)methyl)-, as prepared in Example 4, along with 144 g of glyme. The temperature of this solution is increased to reflux (85° C.) and 28 g of glyme is removed by distillation. To this stirred solution is added 35.8 g (0.12 mol) of polyphosphoric acid under nitrogen. This mixture is stirred vigorously for 12 hours. After 12 hours the reaction mixture is poured into 1000 g of deionized water and a tan colored precipitate is formed. The precipitate is isolated on a Buchner funnel to give 103 g (98% yield) of the title compound, m.p. 50°-58° C. A CDCl3 solution of the product is acidified with TFA-d7 and derivatized with BSTFA. The 31 P-NMR (500 MHz, CDCl3), complex signals at d-18 ppm are consistent with the bistrimethylsilyl ester of 1-propyldihydrogenphosphate, 3-(perfluoro-C6-18 alkyl)oxy)-2,2-bis((perfluoro-C6-19 alkyl)oxy)methyl)-, being the major product. Other signals at -26.4 ppm and -32 ppm are consistent with inorganic phosphorous and pyrophosphate type phosphorous respectively. Signals at -22.6 ppm and -31.3 ppm are consistent with a dimer type structure.
References:
US5491261,1996,A
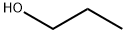
71-23-8
749 suppliers
$12.00/100g

1623-06-9
3 suppliers
inquiry
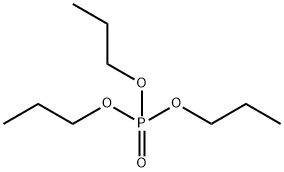
513-08-6
136 suppliers
$50.89/5g
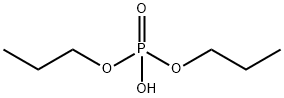
1804-93-9
2 suppliers
inquiry