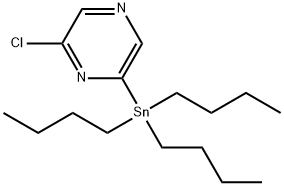
Pyrazine, 2-chloro-6-(tributylstannyl)- synthesis
- Product Name:Pyrazine, 2-chloro-6-(tributylstannyl)-
- CAS Number:850221-68-0
- Molecular formula:C16H29ClN2Sn
- Molecular Weight:403.58
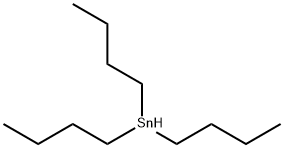
14508-49-7
373 suppliers
$14.00/25g

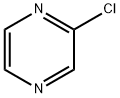
1461-22-9
213 suppliers
$10.00/10g

850221-68-0
3 suppliers
inquiry
Yield:850221-68-0 71.5%
Reaction Conditions:
with 2,2,6,6-tetramethyl-piperidine;n-butyllithium in tetrahydrofuran;hexane at -78 - -40;
Steps:
4
Preparation of compound 4a: 2-chloro-6-(tributylstannyl)pyrazineTo a solution of nBuLi, 1.6m in hexane (21.49 mL, 34.4 mmol) in THF (80 mL) at -50 °C was added 2,2,6,6-tetramethylpiperidine (5.80 mL, 34.4 mmol). The mixture was warmed to 0 °C and stirred for 20 min. The mixture was then cooled to -78 °C. In another flask, 2-chloropyrazine (0.974 mL, 10.91 mmol) and tributyltin chloride (2.96 mL, 10.91 mmol) were dissolved in THF (50 mL) and cooled to -78 °C. The cooled solution was transferred to lithium solution through cannula and the resulting orange mixture was slowly warmed up to -40 °C in 3 h, and was quenched with sat.HCl:EtOH:THF (1 :4:5, 50 mL) and warmed to RT. The mixture was neutralized with sat. NaHCO^ and the volatiles were removed. The residue was diluted in H20 and extracted with DCM. The combined organic layers were dried, filtered and concentrated. The residue was purified with silica gel chromatography (eluting with 0-30% DCM in Hex) to give 2-chloro-6-(tributylstannyl)pyrazine (3.15 g, 7.81 mmol, 71.5 % ) as a clear oil. MS (ESI, pos. ion) m/z: 405 (M+l). .H NMR (400 MHz, CDCl3) δ ppm 8.42 (1 H, s), 8.38 (1 H, s), 1.50 - 1.62 (6 H, m), 1.27 - 1.41 (6 H, m), 1.14 - 1.22 (6 H, m), 0.89 (9 H, t, J=7.3 Hz)
References:
WO2012/129338,2012,A1 Location in patent:Page/Page column 74-75