
Pyrazolo[1,5-a]pyridine, 2-(1-methylethyl)- (9CI) synthesis
- Product Name:Pyrazolo[1,5-a]pyridine, 2-(1-methylethyl)- (9CI)
- CAS Number:59942-84-6
- Molecular formula:C10H12N2
- Molecular Weight:160.22
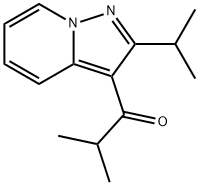
50847-11-5
319 suppliers
$8.00/10mg
![Pyrazolo[1,5-a]pyridine, 2-(1-methylethyl)- (9CI)](/CAS/GIF/59942-84-6.gif)
59942-84-6
8 suppliers
inquiry
Yield:59942-84-6 99%
Reaction Conditions:
Stage #1: ibudilastwith sulfuric acid in water at 135;Inert atmosphere;
Stage #2: with sodium hydroxide in water at 25;
Steps:
1.1.1
Step 1. Method 1. Preparation of Isopropylpyrazolo[1,5-a]pyridine (IPPP) from ibudilast A 5 L 3-neck round-bottom flask was equipped with a mechanical stirrer, thermocouple, heating mantle and a Y-adapter with a nitrogen inlet. The flask was charged with water (350 mL, USP), concentrated sulfuric acid (350 mL) and ibudilast (3-isobutyryl-2-isopropylpyrazolo[1,5-a]pyridine) (140 g, 0.608 mol). The flask was purged with nitrogen, and the mixture was stirred while it was heated to 135° C. An aliquot was removed for HPLC analysis, which showed that all starting material was consumed after 5 hours at 135° C., so the mixture was allowed to cool to room temperature overnight. The mixture was cooled in an ice bath, and water (1400 mL, USP) was added over 10 min, with the temperature maintained below 25° C. With continuous cooling in an ice bath, the mixture was neutralized by adding sodium hydroxide (50% w/w aq., 1150 mL) dropwise, with the temperature maintained below 25° C. Ethyl acetate (250 mL) was added, and the layers were separated. The aqueous layer was washed with ethyl acetate (2×300 mL). The combined ethyl acetate extracts were washed sequentially with 250 mL portions of saturated aqueous sodium bicarbonate and saturated aqueous sodium chloride, then dried over anhydrous sodium sulfate for 30 minutes. Activated carbon (20 g) and silica (60 g) were added and stirred before filtering over a pad of Celite. The filtrate was concentrated under reduced pressure to obtain 96.5 g of IPPP (2-isopropyl-pyrazolo[1,5-a]pyridine, 99% crude yield, 99.6 area % pure by HPLC) as an amber oil.1H-NMR (CDCl3) δ 1.4 (d, 6H), 3.2 (m, 1H), 6.3 (s, 1H), 6.6 (t, 1H), 7.0 (m, 1H), 7.4 (d, 1H), 8.4 (d, 1H). HPLC: RT=9.1 min (99.6 area %).
References:
US2010/324082,2010,A1 Location in patent:Page/Page column 9-10
![Pyrazolo[1,5-a]pyridine,2-(1-methylethenyl)-](/CAS/20180703/GIF/141418-09-9.gif)
141418-09-9
1 suppliers
inquiry
![Pyrazolo[1,5-a]pyridine, 2-(1-methylethyl)- (9CI)](/CAS/GIF/59942-84-6.gif)
59942-84-6
8 suppliers
inquiry
![2-Isopropylpyrazolo[1,5-a]pyridine-3-carboxylic acid](/CAS/20150408/GIF/126959-38-4.gif)
126959-38-4
11 suppliers
inquiry
![Pyrazolo[1,5-a]pyridine, 2-(1-methylethyl)- (9CI)](/CAS/GIF/59942-84-6.gif)
59942-84-6
8 suppliers
inquiry
7583-90-6
85 suppliers
$91.00/1g
97-72-3
215 suppliers
$10.00/10g

50847-11-5
319 suppliers
$8.00/10mg
![Pyrazolo[1,5-a]pyridine, 2-(1-methylethyl)- (9CI)](/CAS/GIF/59942-84-6.gif)
59942-84-6
8 suppliers
inquiry
10330-59-3
12 suppliers
inquiry
![Pyrazolo[1,5-a]pyridine, 2-(1-methylethyl)- (9CI)](/CAS/GIF/59942-84-6.gif)
59942-84-6
8 suppliers
inquiry