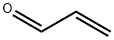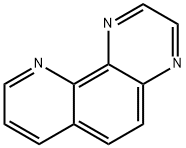
Pyrido[2,3-f]quinoxaline synthesis
- Product Name:Pyrido[2,3-f]quinoxaline
- CAS Number:231-21-0
- Molecular formula:C11H7N3
- Molecular Weight:181.19
Yield:231-21-0 81%
Reaction Conditions:
in methanol at 20; for 1.5 h;
Steps:
Pyrido[2,3-f]quinoxaline (8)
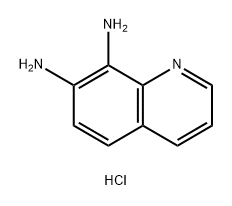
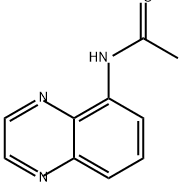
Pyrido[2,3-f]quinoxaline (8). Diimine 7 (113 mg, 0.51 mmol) was added to a stirred solution of hydrochloride 5 (100 mg,0.51 mmol) in MeOH (8 mL) and stirring was continued at room temperature for 1.5 h. The volatiles were evaporated under reduced pressure and the residue was purified by FLC (silica gel, CHCl3/MeOH 100:1, Rf = 0.25) to afford pyridoquinoxaline 8 (75 mg, 81%) as an off-white solid; mp 147-149 °C (Ref. [16] mp 146.5-147.5 °C). 1H NMR (300MHz, CDCl3) δ 7.69 (dd, 1H, J = 8.1, 4.3 Hz, H-8), 8.03 (d, 1H,J = 9.1 Hz, H-5), 8.08 (d, 1H, J = 9.1 Hz, H-6), 8.30 (dd, 1H, J= 8.1, 1.6 Hz, H-7), 9.01 (d, 1H, J = 1.9 Hz, H-3), 9.10 (d, 1H, J= 1.9 Hz, H-2), 9.21 (dd, 1H, J = 4.3, 1.6 Hz, H-9); 13C NMR(75 MHz, CDCl3) δ 123.6, 128.1, 128.4, 130.1, 136.1, 141.3,144.4, 144.5, 145.5, 145.7, 150.7; anal. calcd for C11H7N3: C,72.92; H, 3.89; N, 23.19; found: C, 72.97; H, 3.90; N, 23.17.
References:
Bella, Maros;Milata, Viktor [Beilstein Journal of Organic Chemistry,2013,vol. 9,p. 2669 - 2674]
![[1,2,5]Selenadiazolo[3,4-h]quinolin-6(9H)-one](/CAS/20210305/GIF/1254106-91-6.gif)
1254106-91-6
0 suppliers
inquiry
![Pyrido[2,3-f]quinoxaline](/CAS/20180629/GIF/231-21-0.gif)
231-21-0
4 suppliers
inquiry
![[1,2,5]Selenadiazolo[3,4-h]quinoline, 6-chloro-](/CAS/20210305/GIF/1609035-44-0.gif)
1609035-44-0
0 suppliers
inquiry
![Pyrido[2,3-f]quinoxaline](/CAS/20180629/GIF/231-21-0.gif)
231-21-0
4 suppliers
inquiry
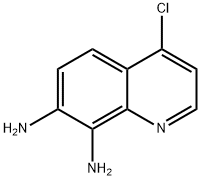
1609035-45-1
0 suppliers
inquiry
![Pyrido[2,3-f]quinoxaline](/CAS/20180629/GIF/231-21-0.gif)
231-21-0
4 suppliers
inquiry