
Pyrimidine, 5-(2-chloroethyl)- synthesis
- Product Name:Pyrimidine, 5-(2-chloroethyl)-
- CAS Number:1248638-20-1
- Molecular formula:C6H7ClN2
- Molecular Weight:142.59

875251-47-1
75 suppliers
$155.00/25mg

1248638-20-1
2 suppliers
inquiry
Yield:1248638-20-1 77%
Reaction Conditions:
with thionyl chloride in dichloromethane at 0 - 50;Inert atmosphere;
Steps:
A.xxxix Synthesis of 5-(2-chloroethyl) pyrimidine:
[0715] Synthesis of 5-(2-chloroethyl) pyrimidine: [0716] To a stirred solution of 2-(pyrimidin-5-yl) ethanol (20 mg, 0.16 mmol) in CH2CI2 (4 mL) under argon atmosphere was added sulfurous dichloride (0.02 mL, 0.32 mmol) at 0 °C; heated to 50 °C and stirred for 4 h. The reaction was monitored by TLC; after completion of the reaction, the reaction mass was cooled to room temperature and pH was adjusted to ~8 using saturated NaHC03 solution( 5 mL) and the compound was extracted with CH2CI2 (2 x 10 mL). The combined organic extracts were dried over sodium sulphate, filtered and concentrated in vacuo to obtain the crude. The crude was purified through silica gel column chromatography using 2% MeOH/ CH2C12 to afford 5-(2-chloroethyl) pyrimidine (18 mg, 77%) as colorless syrup. [0717] 1H-NMR (CDC13, 400 MHz): δ 9.16 (s, 1H), 8.66 (s, 2H), 3.78 (t, 2H), 3.12 (t, 2H); LC-MS: 97.54%; 143 (M++l); (column; Xbridge C-18, 50 3.0 mm, 3.5 μπι); RT 1.80 min. 0.05% TFA (aq.): ACN; 0.8 mL/min); UPLC (purity): 99.22%; (column: Acquity BEH C-18, 50 x 2.1 mm, 1.7 μ); RT 1.15 min. ACN : 0.025% TFA (Aq); 0.5 mL/min. (IP12060335); TLC: 10% MeOH/ CH2C12 (Rf: 0.7).
References:
WO2013/142269,2013,A1 Location in patent:Paragraph 0715; 0716; 0717
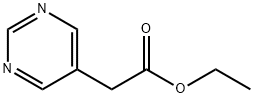
6214-48-8
28 suppliers
$377.00/1g

1248638-20-1
2 suppliers
inquiry