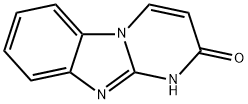
Pyrimido[1,2-a]benzimidazol-2(1H)-one (8CI,9CI) synthesis
- Product Name:Pyrimido[1,2-a]benzimidazol-2(1H)-one (8CI,9CI)
- CAS Number:24811-78-7
- Molecular formula:C10H7N3O
- Molecular Weight:185.18
![2-(trichloromethyl)benzo[4,5]imidazol[1,2-a]pyrimidine](/CAS/20180601/GIF/1320212-46-1.gif)
1320212-46-1
2 suppliers
inquiry
![Pyrimido[1,2-a]benzimidazol-2(1H)-one (8CI,9CI)](/CAS/GIF/24811-78-7.gif)
24811-78-7
10 suppliers
inquiry
Yield:24811-78-7 99%
Reaction Conditions:
with sodium hydroxide in acetonitrile; for 0.5 h;Reflux;
Steps:
(d). Benzo[4,5]imidazo[1,2-a]pyrimidin-2-ol (4)
NaOH (1N, 42mL, 42mmol) was added to a mixture containing compound 3 (9.16g, 32.0mmol) in acetonitrile (160mL). The reaction was heated to reflux for 30min, and then the reaction was cooled and concentrated. Added ice to the resulting residue, followed by HCl (1N, 30mL) to adjust pH of the solution to 8. Filtered the solid and dried in air to afford 4 (5.87g, 99%) as a white solid, Rf=0.16 (10% MeOH/CH2Cl2), mp >280°C (decomposed). 1H NMR (DMSO-d6): δ 6.09 (d, J=7.5Hz, 1H, Ar-H), 7.23 (dt, J=1.0, 8.0Hz, 1H, Ph-H), 7.29 (dt, J=1.0, 8.0Hz, 1H, Ph-H), 7.51 (d, J=7.5Hz, 1H, Ph-H), 7.89 (d, J=7.5Hz, 1H, Ph-H), 8.78 (d, J=8.0Hz, 1H, Ar-H), 12.57 (s, OH).
References:
Gao, Mingzhang;Wang, Min;Zheng, Qi-Huang [Bioorganic and Medicinal Chemistry Letters,2014,vol. 24,# 1,p. 254 - 257]

934-32-7
318 suppliers
$6.00/5g
![Pyrimido[1,2-a]benzimidazol-2(1H)-one (8CI,9CI)](/CAS/GIF/24811-78-7.gif)
24811-78-7
10 suppliers
inquiry