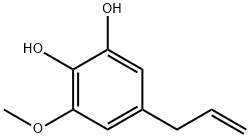
Pyrocatechol synthesis
- Product Name:Pyrocatechol
- CAS Number:4055-72-5
- Molecular formula:C10H12O3
- Molecular Weight:180.2
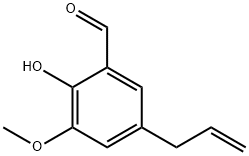
22934-51-6
61 suppliers
$45.00/50mg

4055-72-5
4 suppliers
inquiry
Yield:-
Reaction Conditions:
with pyridine;dihydrogen peroxide;sodium hydroxide in water at 20; for 1.5 h;
Steps:
1.2 Stage 2 - preparation of5-Hydroxy-eugenol (L)i)
Stage 2 - preparation of5-Hydroxy-eugenol (L)i)To a solution of eugenol-5-aldehyde (2.5 g) in pyridine (16 cc) was added one normal aqueous sodium hydroxide (19.5 cc) and the clear yellow solution treated dropwise with 6% hydrogen peroxide (9.6 cc) with vigorous shaking. The colour changed to red and when the solution developed turbidity a little more water was added in order toremove it. After leaving at the room temperature for 1.5 hours with occasional shaking, the solution was acidified with hydrochloric acid while cooling. The hydroxy compound separated as a heavy liquid; this separation was completed by adding common salt and the mixture was extracted thrice with ether. The ether extract was washed successively with hydrochloric acid, aqueous sodium bicarbonate and a smallquantity of water. After drying over sodium sulphate it was evaporated to remove the solvent completely; a pale brown viscous oil was left behind (2.0 g). The product fromthree experiments was collected and distilled under reduced pressure (20 mm); the main fraction distilled at 176°. 5-hydroxy eugenol was a colourless viscous liquid. It was soluble in 5% aqueous sodium hydroxide and 10% aqueous sodium carbonate to give a deep brown solution. With a drop of ferric chloride in alcoholic solution it gave adeep violet colour changing to brownish violet and brown with another drop. After standing for a few minutes the colour changed to a stable olive green. It gave a yellow precipitate with lead acetate.
References:
WO2013/133723,2013,A1 Location in patent:Page/Page column 18; 19

934-00-9
170 suppliers
$32.70/5g

4055-72-5
4 suppliers
inquiry
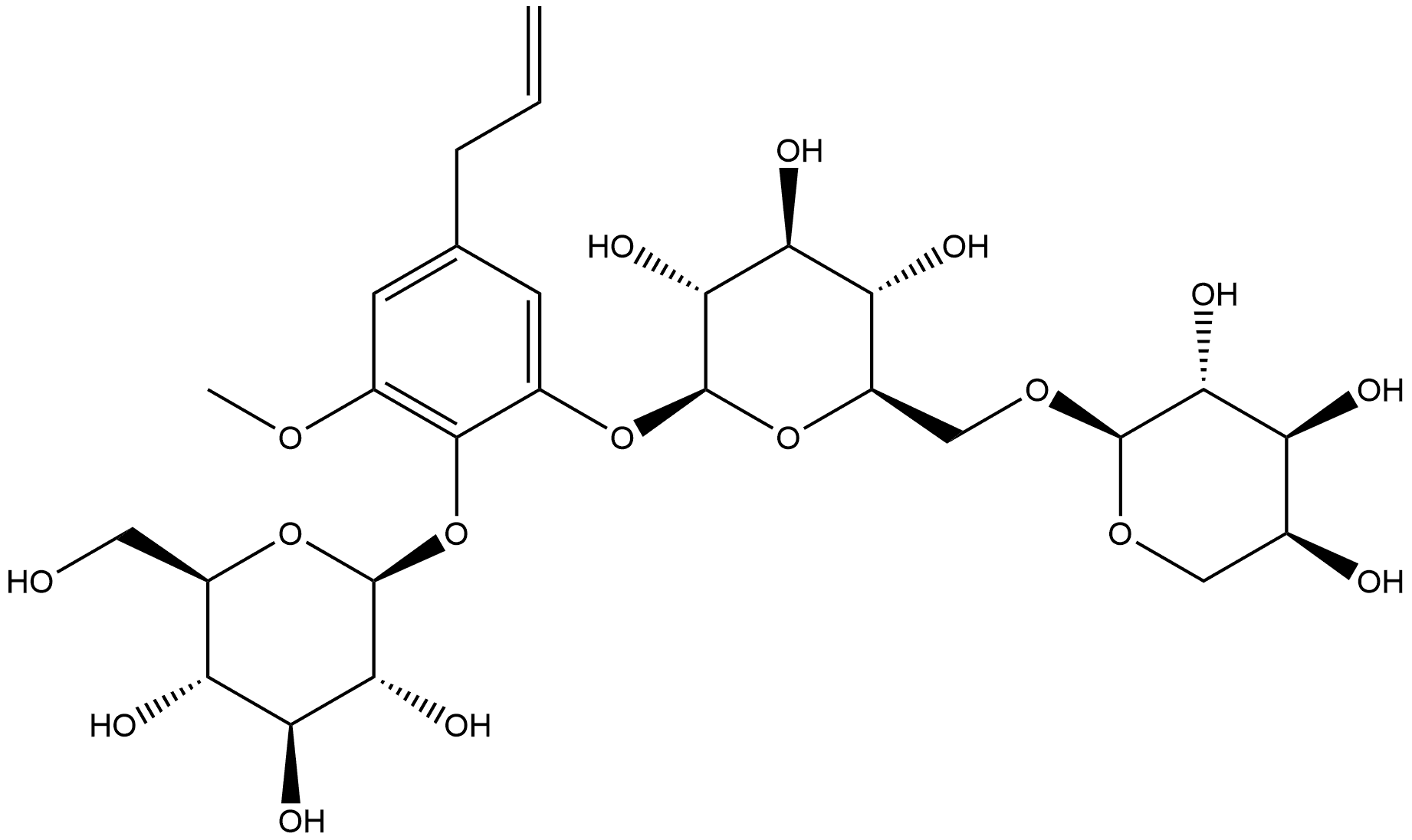
138614-64-9
0 suppliers
inquiry

4055-72-5
4 suppliers
inquiry
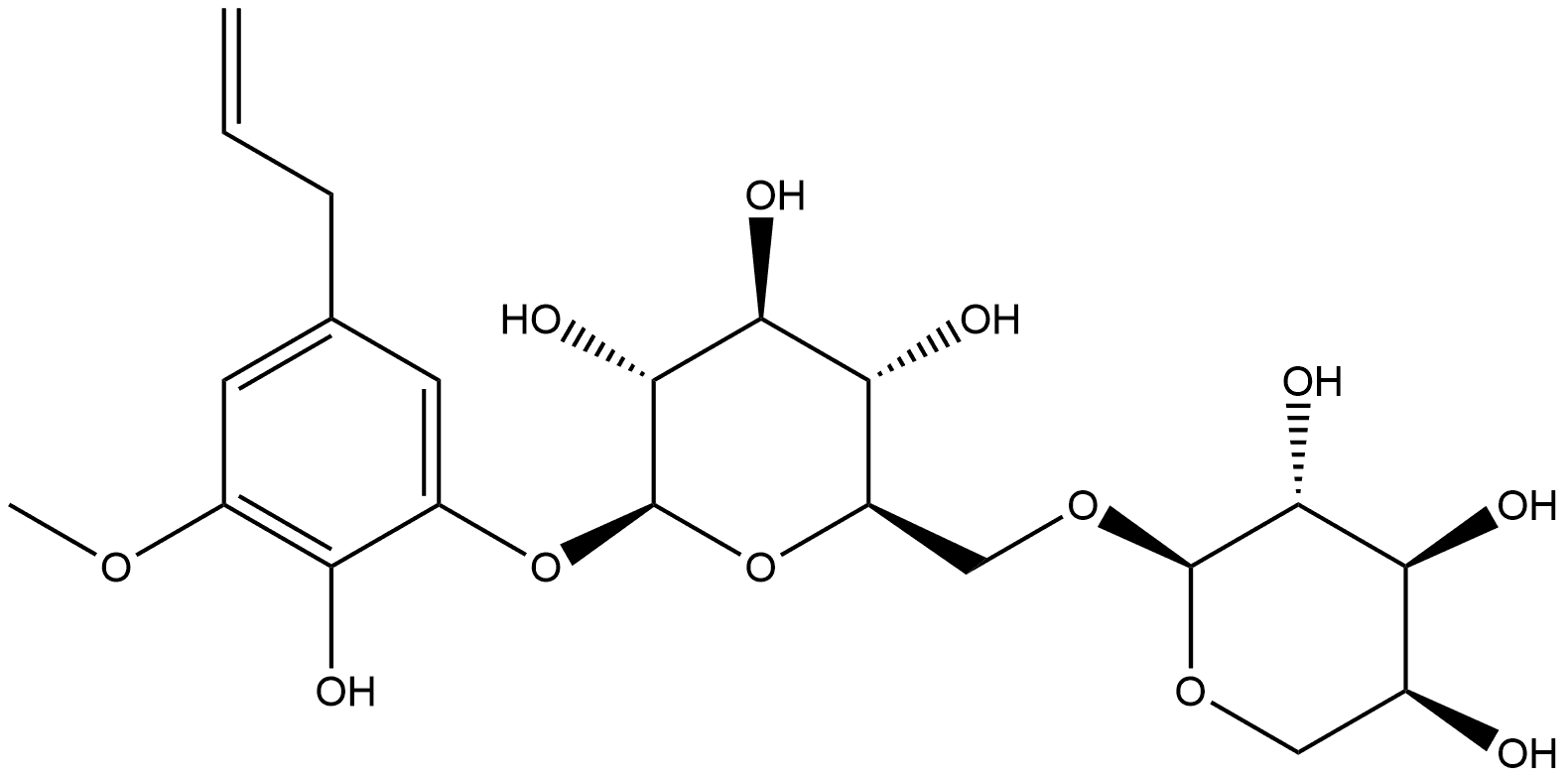
138614-63-8
0 suppliers
inquiry

4055-72-5
4 suppliers
inquiry
138614-62-7
0 suppliers
inquiry

4055-72-5
4 suppliers
inquiry