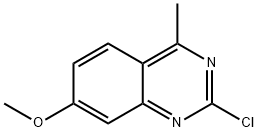
Quinazoline, 2-chloro-7-methoxy-4-methyl- synthesis
- Product Name:Quinazoline, 2-chloro-7-methoxy-4-methyl-
- CAS Number:58487-56-2
- Molecular formula:C10H9ClN2O
- Molecular Weight:208.64

37528-39-5
2 suppliers
inquiry

58487-56-2
8 suppliers
inquiry
Yield:58487-56-2 95.9%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;trichlorophosphate in toluene at 80; for 6 h;
Steps:
Synthesis of 2-chloro-7-methoxy-4-methyl-quinazoline
Synthesis of 2-chloro-7-methoxy-4-methyl-quinazoline BCI3 (40.65 mL, 0.046mol, 1 M solution in toluene) was added dropwise toa solution of compound 6 (5.0 g, 0.046 mol)in dry toluene (50 mL) at -5°C over a periodof 1 hour. MeCN (2.26 g 0.055 mol) was then added dropwise at 0-5°C over a period of 10min, the mixture was stirred at 0°C for 40 min, solid AICI3 (6.73 g, 0.051 mol)was added at 0-5°C. The mixture was stirred for 2 hours at 25 °C and heated toreflux for 20 hours. The mixture was cooled to 0°C and quenched by adding /'-PrOH (30 mL) and H2O (50 mL) at 0-5°C. The mixture was stirred for 2 hours and reflux for3h and cooled to r.t. The mixture was basified to pH = 3 with 25% aq. NaOH, andextracted with CH2CI2 (50 mL x 3), the organic layers were concentratedin vacuum, the residue was washed by MTBE to give compound 6a (2.1 g, yield: 31.3%). m/z = 165 [M +H]+ .To a solution of the compound 6a (3.0g, 18.2 mmol) in 30 mL of THF was added DMAP (2.18 g, 21 .8mmol) and CI3CCOCI(3.31 g, 18.2 mmol) at 0°C, then the mixture was stirred at room temperaturefor 6 hours. The resulting mixture was diluted with ice water (50 mL),extracted with EtOAc (20 mL χ 3), the organiclayers were concentrated in vacuum, the residue was purified by chromatographyon silica gel (PE / EtOAc = 20: 1 ) to give compound 6b (3.01 g, yield: 53.6%).To a solution of the compound 6b (1.0 g, 3.2 mmol) in 15 mL of DMSO was added CH3COONH4 (1 .242 g, 16.1 mmol), themixture was stirred at room temperature for 20 hours. The resulting mixture wasdiluted with cold H2O, the formedprecipitate was collected by filtration and dried under vacuum to affordcompound 7-methoxy-4-methyl-1 H-quinazolin-2-one (0.25 g, yield: 41 .1 %),which was used for next step without further purification, m/z = 191 [M + H]+ .To a solution of the compound7-methoxy-4-methyl-1 H-quinazolin-2-one (300 mg, 1 .56 mmol) in 10 mL of toluene was added POCI3 (405.8 mg, 2.65mmol) and DIPEA (402.5 mg, 3.12 mmol), then the mixture was stirred at 80°C for6 hours. The resulting mixture was poured into 10mL of K2CO3, extracted with CH2CI2 (5 mL x 3), the organic layers wereconcentrated in vacuum to give 2- chloro-7-methoxy-4-methyl-quinazoline (291mg, yield: 95.9%).
References:
WO2013/50527,2013,A1 Location in patent:Page/Page column 48; 49; 50

37528-39-5
2 suppliers
inquiry

58487-56-2
8 suppliers
inquiry
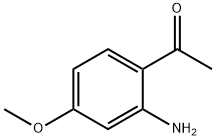
42465-53-2
99 suppliers
$45.00/25mg

58487-56-2
8 suppliers
inquiry

536-90-3
309 suppliers
$10.00/5g

58487-56-2
8 suppliers
inquiry
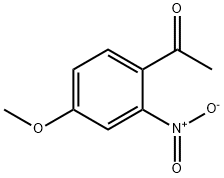
67323-06-2
14 suppliers
inquiry

58487-56-2
8 suppliers
inquiry