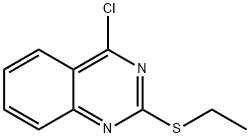
Quinazoline, 4-chloro-2-(ethylthio)- synthesis
- Product Name:Quinazoline, 4-chloro-2-(ethylthio)-
- CAS Number:58803-78-4
- Molecular formula:C10H9ClN2S
- Molecular Weight:224.71
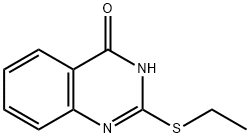
16802-73-6
6 suppliers
inquiry

58803-78-4
13 suppliers
inquiry
Yield:58803-78-4 79%
Reaction Conditions:
Stage #1: 2-ethylsulfanyl-3H-quinazolin-4-onewith thionyl chloride;N,N-dimethyl-formamide for 3 - 4 h;Heating / reflux;
Stage #2: with water;sodium carbonate
Steps:
2
Example 2 Preparation of 4-chloro-2-ethylsulfanylquinazoline (formula 2a) To 2-ethylsulfanyl-3H-quinazolino-4-one (formula B; 1.5 g, 7.27 mmol) was slowly added 50 mL of thionyl chloride at 0° C. After adding 2-3 drops of N,N-dimethylformamide, reflux was performed for 3-4 hours. When the starting materials disappeared, thionyl chloride was removed by distillation under reduced pressure. Residual thionyl chloride was washed with a saturated sodium carbonate solution. The mixture was extracted with ethyl acetate and the organic layer was washed with brine and dried with anhydrous magnesium sulfate. The organic solvent was removed by distillation under reduced pressure and the target compound was obtained with a yield of 79% (1.29 g) by column chromatography. 1H NMR (CDCl3, 300 MHz) δ 8.02 (d, J=7.8 Hz, 1H), 7.85 (m, 2H), 7.56 (t, J=7.5 Hz, 1H), 3.29 (q, J=14.4 Hz, 2H), 1.49 (t, J=7.35 Hz, 3H).
References:
US2008/207614,2008,A1 Location in patent:Page/Page column 15-16
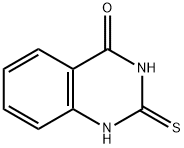
13906-09-7
82 suppliers
$45.00/250mg

58803-78-4
13 suppliers
inquiry