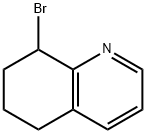
Quinoline, 8-bromo-5,6,7,8-tetrahydro- synthesis
- Product Name:Quinoline, 8-bromo-5,6,7,8-tetrahydro-
- CAS Number:125163-08-8
- Molecular formula:C9H10BrN
- Molecular Weight:212.09
10500-57-9
285 suppliers
$6.00/5g

125163-08-8
1 suppliers
inquiry
Yield:125163-08-8 66%
Reaction Conditions:
with N-Bromosuccinimide;azobisisobutyronitrile in tetrachloromethane at 20; for 12 h;Irradiation;
Steps:
8-Bromo-5,6,7,8-tetrahydroquinoline (2x):
General procedure: To a rt solution of 2-propylpyridine (2.5 g, 20.63 mmol) and (E)-2,2'-(diazene-1,2-diyl)bis(2-methylpropanenitrile)(1.253 g, 7.63 mmol) in CCl4 (60 mL) was added N-bromosuccinimide(1.933 mL, 22.69 mmol). The mixture was stirred under fluorescent light at rt for 12 h. The precipitates were filtered off through a pad of celite, washed with CCl4 (10 mL). The organic solutions were combined. After removal of the organic solvent under reduced pressure, purification of the residue by flash chromatography on silica gel with 0-20% EtOAc/Hexanes provided the title compound 2d as a pale yellow liquid (3.18 g, 77%).
References:
Yu, Ming;Stevenson, Karis;Zhou, Gene [Tetrahedron Letters,2014,vol. 55,# 41,p. 5591 - 5594] Location in patent:supporting information
14631-46-0
117 suppliers
$10.00/250mg

125163-08-8
1 suppliers
inquiry