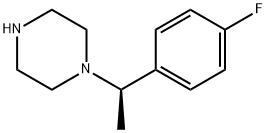
(R)-1-(1-(4-FLUOROPHENYL)ETHYL)PIPERAZINE synthesis
- Product Name:(R)-1-(1-(4-FLUOROPHENYL)ETHYL)PIPERAZINE
- CAS Number:862270-48-2
- Molecular formula:C12H17FN2
- Molecular Weight:208.28
![Piperazine, 1-[(1R)-1-(4-fluorophenyl)ethyl]-4-[(4-methylphenyl)sulfonyl]-](/CAS/20210305/GIF/862270-47-1.gif)
862270-47-1
0 suppliers
inquiry

862270-48-2
30 suppliers
$45.00/10mg
Yield:-
Reaction Conditions:
Stage #1: 1-[(R)-1-(4-fluorophenyl)ethyl]-4-(toluene-4-sulfonyl)piperazinewith hydrogen bromide;acetic acid;4-hydroxy-benzoic acid at 20; for 48 h;
Stage #2: with water at 20; for 2 h;
Stage #3: with potassium hydroxide in water at 0; pH=> 10;
Steps:
23.c
A mixture of 1-[(1R)-1-(4-fluoro-phenyl)-ethyl]-4-(toluene-4-sulfonyl)-piperazine, Example 23(b), (20 g, 55 mmol), 4-hydroxybenzoic acid (22.9 g, 166 mmol, Aldrich) and HBr solution in AcOH (33 wt %, 200 mL, Aldrich) was stirred at room temperature under nitrogen atmosphere for 48 h. Water (200 mL) was added slowly and the mixture was stirred for 2 h at room temperature. The solid precipitate was filtered and the filter cake was washed with H2O (2×50 mL). The filtrate and the H2O washes were combined and extracted with toluene (4×50 mL). The aqueous phase was cooled in an ice bath and treated portionwise with solid KOH (235 g) until pH>10. The aqueous solution was extracted with toluene (3×50 mL) and ethyl acetate (50 mL). The combined organic extracts were washed with brine (100 mL), dried over MgSO4, filtered, and concentrated under reduced pressure. The residue was dried in vacuo to yield the title compound as a pale-brown solid. MS (ESI, pos. ion.) m/z: 209 (M+1).
References:
US2005/176726,2005,A1 Location in patent:Page/Page column 15-16
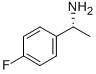
374898-01-8
108 suppliers
$13.00/250mg

862270-48-2
30 suppliers
$45.00/10mg
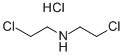
821-48-7
545 suppliers
$6.00/25g

374898-01-8
108 suppliers
$13.00/250mg

862270-48-2
30 suppliers
$45.00/10mg