
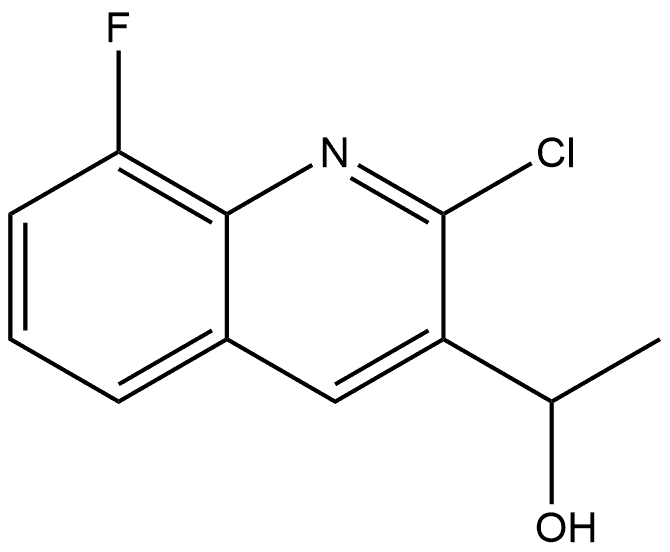
(R)-1-(2-Chloro-8-fluoroquinolin-3-yl)ethanol synthesis
- Product Name:(R)-1-(2-Chloro-8-fluoroquinolin-3-yl)ethanol
- CAS Number:1065481-27-7
- Molecular formula:C11H9ClFNO
- Molecular Weight:225.6467
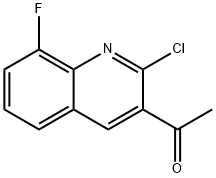
1065481-26-6
6 suppliers
inquiry

1065481-27-7
14 suppliers
$201.00/100mg
Yield:1065481-27-7 73%
Reaction Conditions:
with B-chlorodiisopinocampheylborane in tetrahydrofuran at -55 - 20;
Steps:
1 (R)-1-(2-chloro-8-fluoroquinolin-3-yl)ethanol
In a round bottomed flask was dissolved (+)-dip-chloride(tm) (17.5 g, 540 mmol, 2.2 eq) in anhydrous THF (200 mL) and the solution was cooled to -55° C. (using a dry ice/ MeCN bath). To this solution was added 1-(2-chloro-8-fluoroquinolin-3-yl)ethanone (5.50 g, 24.5 mmol) as a solution in THF (50 mL). The reaction was allowed to warm to rt slowly overnight. After this time the reaction was quenched with 10 mL acetone and 100 mL of 10% Na2CO3 and allowed to stir for 2 h at rt. Ethyl acetate (750 mL) was added and the layers were separated. The organic phase was washed 3× with a 50% saturated sodium bicarbonate solution and once with brine. The organic layer was dried over MgSO4, filtered, and concentrated. The crude material was concentrated under high vacuum at 75° C. to remove pinene. The residue was slurried in 150 mL of hexanes and 150 mL of water for 3 h at rt. A white precipitate formed and was filtered and dried to afford 4.9 g of 98% ee product. The solid was dissolved in 25 mL of boiling EtOAc and 25 mL of hot hexanes was added to form a precipitate at reflux. The mixture was cooled to -15° C., filtered, and washed with cold 9:1 hexanes:EtOAc to afford (R)-1-(2-chloro-8-fluoroquinolin-3-yl)ethanol (4.07 g, 73% yield). Chiral HPLC (10% IPA in hexanes, chiralcel AD-H shows product to be >99.9% ee. Desired enantiomer elutes at 9.6 min, undesired enantiomer elutes at 8.1 min. 1H NMR (400 MHz, CDCl3) δ ppm 8.43 (br s), 7.64 (br d, J=8.2 Hz, 1H), 7.50 (td, J=7.8, 4.7 Hz, 1H), 7.41 (ddd, J=10.2, 7.8, 1.2 Hz, 1H), 5.40 (qd, J=5.9, 0.8 Hz, 1H), 2.22 (br s, 1H), 1.62 (d, J=6.3 Hz, 3H). Mass Spectrum (ESI) m/e=226.0 (M+1).
References:
US2013/267524,2013,A1 Location in patent:Paragraph 0065-0066

1065481-24-4
9 suppliers
inquiry

1065481-27-7
14 suppliers
$201.00/100mg
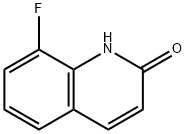
71738-83-5
52 suppliers
$24.00/100mg

1065481-27-7
14 suppliers
$201.00/100mg
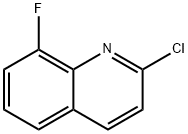
124467-23-8
57 suppliers
$44.50/250mg

1065481-27-7
14 suppliers
$201.00/100mg
1065481-25-5
1 suppliers
inquiry

1065481-27-7
14 suppliers
$201.00/100mg