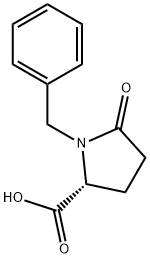
(R)-1-BENZYL-5-CARBOXY-2-PYRROLIDINONE synthesis
- Product Name:(R)-1-BENZYL-5-CARBOXY-2-PYRROLIDINONE
- CAS Number:38854-94-3
- Molecular formula:C12H13NO3
- Molecular Weight:219.24
6893-26-1
456 suppliers
$6.00/10g

100-52-7
941 suppliers
$21.30/2g

38854-94-3
28 suppliers
$150.00/100mg
Yield:38854-94-3 41%
Reaction Conditions:
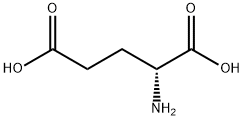
Stage #1: D-Glutamic acid;benzaldehydewith sodium hydroxide in ethanol;water at 20; for 48 h;
Stage #2: with sodium tetrahydroborate in ethanol;water;
Steps:
(2R)-N-(4-Amino-3,4-dioxo-1-phenylbutan-2-yl)-1-benzyl-5-oxopyrrolidine-2-carboxamide(34).
To the solution of (R)-2-aminopentanedioic acid (5.0 g, 34 mmol) in NaOH (34 mL, 2 M in water) benzaldehyde (3.4 mL, 34 mmol) - dissolved in 9 mL of EtOH - was added, and the mixture was stirred at room temperature for 2 days. Then NaBH4(1.54 g, 40.8 mmol) was added in portions and stirring continued until the reaction was compeleted. Water was added, the mixture extracted twice with methyl-tert.butylether, the aqueous layer acidified to pH 3-4 using conc. HCl, and then concentrated to dryness. The white powder obtained was dissolved in EtOH (254 mL) and refluxed for 8 hours. The mixture was filtrated, concentrated in vacuo, the remainderdissolved in aqueous NaOH, extracted 3x with ethyl acetate, the aqueous layer acidified using conc. HCl, and extracted again with dichloromethane. The combined organic layers were washed with brine, dried over Na2SO4, filtered off and concentrated to give the title compound as colorless oil (3.03 g, 41% yield).ESI MS (m/z): calcd for C12H13NO3, 219.2; found 220.1 [M + H+].
References:
Jantos, Katja;Kling, Andreas;Mack, Helmut;Hornberger, Wilfried;Moeller, Achim;Nimmrich, Volker;Lao, Yanbin;Nijsen, Marjoleen [Bioorganic and Medicinal Chemistry Letters,2019,vol. 29,# 15,p. 1968 - 1973] Location in patent:supporting information