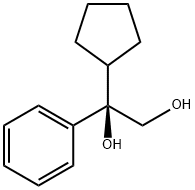
(R)-1-cyclopentyl-1-phenylethane-1,2-diol synthesis
- Product Name:(R)-1-cyclopentyl-1-phenylethane-1,2-diol
- CAS Number:183201-49-2
- Molecular formula:C13H18O2
- Molecular Weight:206.28
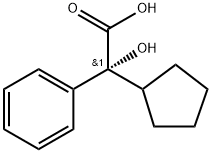
64471-45-0
25 suppliers
inquiry

183201-49-2
15 suppliers
inquiry
Yield:183201-49-2 100%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran; for 3 h;Reflux;
Steps:
1.5 1.5 Synthesis of (R) -a-phenyl-α-cyclopentyl-α-phenylmethyl p-toluenesulfonate (VIII)
(R) -a-phenyl-a-cyclopentyl-a-hydroxyacetic acid (VI) in an amount of 2.2 g (0. Olmol)In 10 mL of anhydrous THF was slowly added dropwise to a 20 mL anhydrous THF solution containing LiAlH4 (0.02 mol), and the temperature was slowly raised with stirringTo reflux, reaction 3h. After cooling, carefully add 2 mL of saturated NaHC03 solution and add 10 mL of 2N NaOH solution. IsolationThe aqueous phase was extracted with diethyl ether. The combined organic phases were washed with saturated brine and dried over anhydrous sodium sulfate. Vacuum distillation,The solvent was removed to give 2.06 g of (R) -a-phenyl-α-cyclopentyl-α-hydroxyethanol (VII) as colorless needles,100%,
References:
CN102070631,2016,B Location in patent:Paragraph 0085-0086
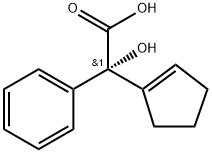
185030-09-5
4 suppliers
inquiry

183201-49-2
15 suppliers
inquiry
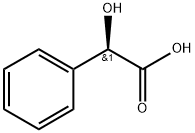
611-71-2
618 suppliers
$13.00/5g

183201-49-2
15 suppliers
inquiry
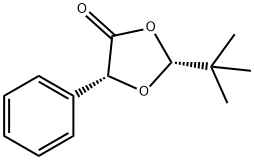
116907-30-3
0 suppliers
inquiry

183201-49-2
15 suppliers
inquiry
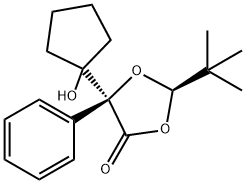
909298-90-4
1 suppliers
inquiry

183201-49-2
15 suppliers
inquiry