
(R)-1-N-Boc-2-(hydroxymethyl)piperazine synthesis
- Product Name:(R)-1-N-Boc-2-(hydroxymethyl)piperazine
- CAS Number:169448-87-7
- Molecular formula:C10H20N2O3
- Molecular Weight:216.28

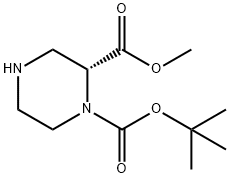
252990-05-9
142 suppliers
$80.00/1G

169448-87-7
127 suppliers
$32.00/100mg
Yield:169448-87-7 100%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at -40 - 20; for 1 h;
Steps:
34
Lithium aluminium hydride (1M in THF, 26 ml) was added to a solution of L-TERT- butyl 2-methyl (2R)-piperazine-1, 2-dicarboxylate (2. 55 g) in THF (70 ml) AT-40°C, then the reaction was warmed to room temperature. The solution was stirred for 1 hour, then cooled to 0°C and quenched by sequential addition of water (1 ml), sodium hydroxide (2N, 1 ml) and then water (2 ml). The resulting slurry was filtered and concentrated in vacuo to give TERT- butyl (2R)-2-(hydroxymethyl) piperazine-1-carboxylate (2.37 g, > 100%); NMR spectrum (DMSO-d6,373K) 1.40 (s, 9H), 2.58 (m, 1H), 2. 82 (M, 3H), 2. 92 (bs, 1H), 2.98 (d, 1H), 3.43 (m, 1H), 3.65 (M, 2H), 3.80 (M, 1H) ; Mass spectrum MH+ 217.
References:
WO2005/26152,2005,A1 Location in patent:Page/Page column 136

930782-89-1
39 suppliers
$269.85/1G

169448-87-7
127 suppliers
$32.00/100mg
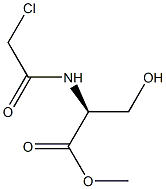
366003-57-8
0 suppliers
inquiry

169448-87-7
127 suppliers
$32.00/100mg
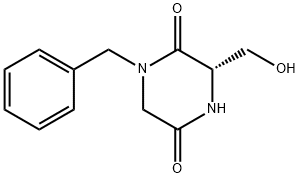
149648-97-5
0 suppliers
inquiry

169448-87-7
127 suppliers
$32.00/100mg
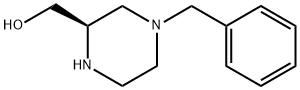
149715-46-8
38 suppliers
$270.31/250mg

169448-87-7
127 suppliers
$32.00/100mg