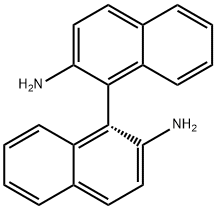
(R)-(+)-2,2'-Diamino-1,1'-binaphthalene synthesis
- Product Name:(R)-(+)-2,2'-Diamino-1,1'-binaphthalene
- CAS Number:18741-85-0
- Molecular formula:C20H16N2
- Molecular Weight:284.35
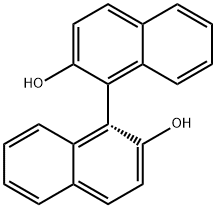
18531-94-7
390 suppliers
$6.00/5g

18741-85-0
159 suppliers
$18.00/250mg
Yield:18741-85-0 82%
Reaction Conditions:
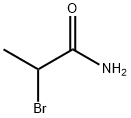
Stage #1: (R)-1,1'-Bi-2-naphtholwith 2-bromo-propionic acid amide;potassium carbonate;potassium iodide in dimethyl sulfoxide at 50; for 24 h;
Stage #2: with potassium hydroxide in dimethyl sulfoxide at 150; for 4 h;
Steps:
38; 59 Example 38: Preparation of (R)-[1,1'-binaphthyl]-2,2'-diamine (4a)
In an air atmosphere, 1a (0.2 mmol), 2b (0.6 mmol) was added to the reaction tube.Potassium carbonate (0.6 mmol), potassium iodide (0.02 mmol), dimethyl sulfoxide (2 ml) as a reaction solvent,After adding a rubber stopper to the reaction tube, it was stirred in an oil bath preheated to 50° C. for 24 hours.Thin layer chromatography (TLC) spots determine the endpoint of the reaction.When the TLC plate shows the reaction material 1aCompletely disappears and is completely converted into a dialkylated product,Lift the reaction tube from the oil bath and directly add potassium hydroxide (2.5 mmol) to the reaction tube.Then put it into an oil bath preheated to 150°C and stir for 4 hours.After the reaction is completed, the reaction tube is cooled to room temperature, and 5 ml of water is added to quench the reaction.Then it was extracted with ethyl acetate and spin-dried, and the target product was isolated by column chromatography.4a.Product yield 82%; yellow solid; 99% ee
References:
CN107814729,2018,A Location in patent:Paragraph 0170-0173; 0254-0257
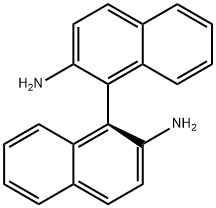
18531-95-8
156 suppliers
$18.00/250mg

18741-85-0
159 suppliers
$18.00/250mg

18531-95-8
156 suppliers
$18.00/250mg

18741-85-0
159 suppliers
$18.00/250mg

18531-95-8
156 suppliers
$18.00/250mg
5875-25-2
70 suppliers
$20.00/1g

18531-94-7
390 suppliers
$6.00/5g

18741-85-0
159 suppliers
$18.00/250mg

18531-94-7
390 suppliers
$6.00/5g

18741-85-0
159 suppliers
$18.00/250mg

137848-28-3
131 suppliers
$50.00/25mg