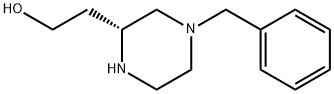
(R)-2-(4-benzylpiperazin-2-yl)ethanol-2HCl synthesis
- Product Name:(R)-2-(4-benzylpiperazin-2-yl)ethanol-2HCl
- CAS Number:857334-79-3
- Molecular formula:C13H20N2O
- Molecular Weight:220.31
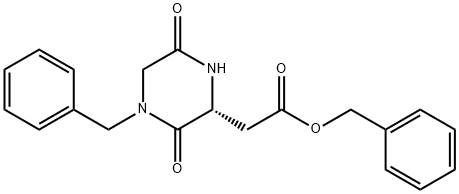
857334-77-1
0 suppliers
inquiry

857334-79-3
9 suppliers
inquiry
Yield: 90%
Reaction Conditions:
Stage #1:(R)-benzyl 2-(4-benzyl-3,6-dioxopiperazin-2-yl)acetate with lithium aluminium tetrahydride in tetrahydrofuran at 20 - 60;Heating / reflux;
Stage #2: with hydrogenchloride in chloroform;water
Steps:
b
A solution of 5. 1g (14.47 mmol) of ( (R)-4-benzyl-3, 6-dioxo-piperazin-2-yl)-acetic acid benzyl ester in 60 mL of dry THF was cautiously added to a reaction flask containing 60 mL of 1 M lithium aluminum hydride in THF stirring under N2. When the addition was complete, the reaction mixture was heated at reflux for 5 h, then kept at 55-60 °C overnight, then refluxed for 7h, then stirred at room temperature overnight. 15 mL of water was cautiously added with vigorous stirring, then the mixture was stirred for 0.5 h. The resulting slurry was vacuum filtered through a fritted glass funnel and the solids were washed with THF and MeOH. The filtrate was concentrated in vacuo and the residue was taken up in CHC13 and extracted twice with 50 mL of 1 N HC1. The aqueous extracts were combined and washed twice with CHC13. The aqueous phase was made basic by addition of a solution of 5 g of NaOH in 50 mL of water. The resulting cloudy alkaline aqueous mixture was extracted twice with 50 mL CHC13. These organic extracts were combined, dried over MgS04, filtered and concentrated in vacuo to give 2. 87g of a colorless oil that slowly solidifies (90%).'H-NMR : 300MHz, CDC13 8 7.4-7. 2 (m, 5H) ; 3.79 (m, 2H); 3.48 (s, 2H); 3.02-2. 78 (m, 3H); 2.77-2. 68 (m, 2H); 2.02 (m, 1H) ; 1.84 (m, 1H) ; 1.58 (m, 2H).
References:
ASTRAZENECA AB WO2005/61510, 2005, A1 Location in patent:Page/Page column 14-15
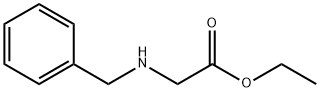
6436-90-4
374 suppliers
$8.00/10g

857334-79-3
9 suppliers
inquiry
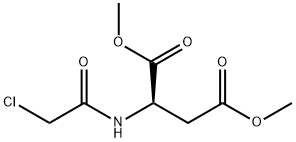
1485770-18-0
0 suppliers
inquiry

857334-79-3
9 suppliers
inquiry