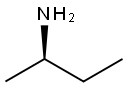
(R)-(-)-2-Aminobutane synthesis
- Product Name:(R)-(-)-2-Aminobutane
- CAS Number:13250-12-9
- Molecular formula:C4H11N
- Molecular Weight:73.14
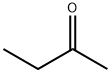
78-93-3
6 suppliers
$30.80/500ml
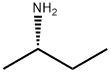
513-49-5
106 suppliers
$50.29/250mg

13250-12-9
105 suppliers
$30.28/100MG
Yield:13250-12-9 6.2 mmol
Reaction Conditions:
with transaminases XP_001209325;rac-methylbenzylamine in aq. phosphate buffer at 28; for 24 h;Enzymatic reaction;enantioselective reaction;Reagent/catalyst;
Steps:
5 Example 5 Synthesis of Enantiomerically Enriched (R)-Amines
General procedure: In a final volume of 0.25 μl buffered with 100 mM potassium phosphate (KPi) at pH 7.5 equimolar amounts of 70 mM of amino donor and 70 mM of ketone substrate were reacted in the presence of 0.1 ml CFE of a transaminase at 28° C. for 24 h. CFE comprising transaminases XP-001209325, AAN21261 and ABN35871, respectively, was incubated with benzylacetone and α-methylbenzylamine yielding 4-phenyl-2-butylamine and acetophenone; propiophenone and α-methylbenzylamine yielding α-ethylbenzylamine and acetophenone; 1-indanone and α-methylbenzylamine yielding 1-aminoindan and acetophenone; 1-tetralone and α-methylbenzylamine yielding 1-aminotetralin and acetophenone; 2-tetralone and α-methylbenzylamine yielding 2-aminotetralin and acetophenone; butanone and α-methylbenzylamine yielding 2-aminobutane and acetophenone; and 3,3-dimethyl-2-butanone and α-methylbenzylamine yielding 3,3-dimethyl-2-aminobutane and acetophenone, respectively. The reactions were stopped by addition of 0.75 ml stopping reagent (50% (v/v) acetonitrile in H2O containing 0.1% (v/v) formic acid) to 0.25 ml reaction volume. The product concentrations and enantiomeric excesses were analysed by HPLC as described in the general part. The results of the HPLC analysis are given in table 6. Further CFE comprising transaminase YP-955297 was incubated with propiophenone and α-methylbenzylamine yielding α-ethylbenzylamine and acetophenone. The reaction was stopped by addition of 0.75 ml stopping reagent (50% (v/v) acetonitrile in H2O containing 0.1% (v/v) formic acid) to 0.25 ml reaction volume. The product concentration and enantiomeric excess was analysed by HPLC as described in the general part. After 24 h incubation at 28° C. 0.87 mmol/l (R)-α-ethylbenzylamine was obtained with an e.e. of 99%. The above results show transaminases XP-001209325 and YP-955297 are highly selective (R)-transaminases. Further it becomes clear that XP-001209325 has a different substrate spectrum than the transaminases AAN21261 and ABN35871: 4-phenyl-2-butylamine was formed in significant concentrations by XP-001209325 but not by transaminases AAN21261 and ABN35871 (table 6). Additionally XP-001209325 produced enantiomerically enriched (R)-2-aminobutane from 2-butanone, while ABN35871 delivered enantiomerically enriched (S)-2-aminobutane (table 6).
References:
DPX HOLDINGS B.V.;SCHURMANN, Martin;PEETERS, Wijnand Peter Helena;SMEETS, Natascha Hubertina Johannes;SCHWAB, Helmut;STEINER, Kerstin;LYPETSKA, Kateryna Mykolayivna;STROHMEIER, Gernot US2016/32335, 2016, A1 Location in patent:Paragraph 0120; 0121; 0122
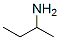
33966-50-6
63 suppliers
inquiry
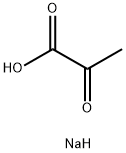
113-24-6
586 suppliers
$13.00/5g

13250-12-9
105 suppliers
$30.28/100MG
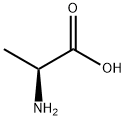
56-41-7
803 suppliers
$5.00/5g

78-93-3
6 suppliers
$30.80/500ml
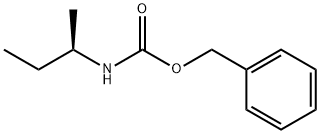
98289-88-4
0 suppliers
inquiry

13250-12-9
105 suppliers
$30.28/100MG

33966-50-6
63 suppliers
inquiry

13250-12-9
105 suppliers
$30.28/100MG
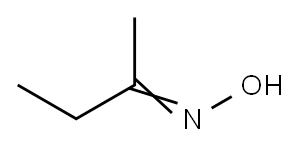
96-29-7
318 suppliers
$13.00/100g

513-49-5
106 suppliers
$50.29/250mg

13250-12-9
105 suppliers
$30.28/100MG