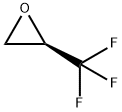
R-(+)-2-TRIFLUOROMETHYLOXIRANE synthesis
- Product Name:R-(+)-2-TRIFLUOROMETHYLOXIRANE
- CAS Number:143142-90-9
- Molecular formula:C3H3F3O
- Molecular Weight:112.05

359-41-1
168 suppliers
$7.00/1g

143142-90-9
38 suppliers
$45.00/10mg
Yield: 76%
Reaction Conditions:
with (1S,2S)-(+)-1,2-cyclohexanediamino-N,N’-bis(3,5-di-t-butylsalicylidene)cobalt (II);acetic acid in water at 0 - 20;
Steps:
1 Preparation 1(2R)-2-(Trifluoromethyl)oxirane
Add acetic acid (0.89 mL, 0.052 eq) to a solution of (1 S,25)-(+)- 1 ,2-cyclohexanediamino-N,N’-bis(3,5-di-t-bu- tylsalicylidene)cobalt (II) (0.90 g, 0.0050 eq) in toluene (16.65 mL). Stir at room temperature for 30 minutes. Remove the solvent in vacuo. Add toluene (20 mL) and concentrate in vacuo. cool to 00 c. and add 2-(trifluorom- ethyl)oxirane (37.00 g, 330 mmol; 80.0% cc, (2R) is the major enantiomer). Stir for five minutes and add water (0.80 mL, 0.15 eq) dropwise. Allow to slowly warm to room temperature and stir overnight. Vacuum distill at room temperature, collecting the title compound in a cooled flask as a light yellow oil (28.10 g, 76%; 99.8% cc). ‘H NMR (CDC13) ö 2.92-2.94 (m, 1H), 2.98-3.01 (m, 1H), 3.41-3.46 (m, 1H).
References:
Eli Lilly and Company;Clayton, Joshua Ryan US9556153, 2017, B1 Location in patent:Page/Page column 7; 8
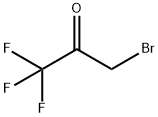
431-35-6
226 suppliers
$10.00/5 g

143142-90-9
38 suppliers
$45.00/10mg

359-41-1
168 suppliers
$7.00/1g
148683-14-1
9 suppliers
inquiry

143142-90-9
38 suppliers
$45.00/10mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
130025-34-2
55 suppliers
$45.00/10mg

143142-90-9
38 suppliers
$45.00/10mg

359-41-1
168 suppliers
$7.00/1g

130025-34-2
55 suppliers
$45.00/10mg

148683-14-1
9 suppliers
inquiry

143142-90-9
38 suppliers
$45.00/10mg