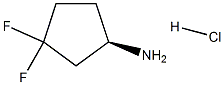
(R)-3,3-DifluorocyclopentanaMine hydrochloride synthesis
- Product Name:(R)-3,3-DifluorocyclopentanaMine hydrochloride
- CAS Number:1117936-64-7
- Molecular formula:C5H10ClF2N
- Molecular Weight:157.5894
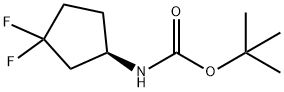
1117936-63-6
2 suppliers
inquiry

1117936-64-7
40 suppliers
$45.00/2.5mg
Yield: 82%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane at 0 - 20;
Steps:
1.4
Hydrochloric acid (4M in dioxane, 60 mL) was added to a solution of (R)-tert- butyl 3,3-difluorocyclopentylcarbamate (4.61 g, 20.8 mmol) in dioxane (40 mL) at 0 0C. After complete addition, the reaction mixture was allowed to warm to room temperature and stirred for 2 hours. The solvent was removed in vacuo and the residue was triturated with diethylether to afford the title compound as an off-white solid (2.68 g, 82% yield). 1H NMR (CDCl3, 400 MHz): δ 1.76-1.88 (IH, m), 2.10-2.40 (4H, m), 2.45-2.60 (IH, m), 3.68 (IH, quint), 8.33 (3H, s).
References:
Location in patent:Page/Page column 43