
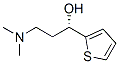
(R)-3-(diMethylaMino)-1-(thiophen-2-yl)propan-1-ol synthesis
- Product Name:(R)-3-(diMethylaMino)-1-(thiophen-2-yl)propan-1-ol
- CAS Number:132335-49-0
- Molecular formula:C9H15NOS
- Molecular Weight:185.29
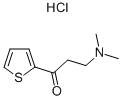
5424-47-5
209 suppliers
$9.00/5g

132335-49-0
34 suppliers
inquiry
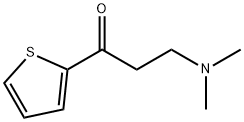
132335-44-5
334 suppliers
$6.00/1g
Yield:132335-49-0 97%
Reaction Conditions:
Stage #1: 3-(dimethylamino)-1-(thiophen-2-yl)propan-1-one hydrochloridewith C36H28FeMnN2O3P(1+)*Br(1-);potassium carbonate in ethanol at 20; for 0.0833333 h;Glovebox;
Stage #2: with hydrogen in ethanol at 50; under 22502.3 Torr; for 16 h;Glovebox;Autoclave;
Steps:
18 Example 18 Asymmetric hydrogenation of 3-(dimethylamino)-1-(2-thienyl)-1-acetone hydrochloride catalyzed by Mn-PNN catalyst
In the glove box, add Mn-Cat.1 catalyst (3.8mg, 0.005mmol) and substrate 3-(dimethylamino)-1-(2-thienyl)-1-acetone hydrochloride (1mmol) into 5mL transparent Inside the glass vial, then potassium carbonate (139.6 mg, 1.01 mmol) and absolute ethanol (3 mL) were added, and the mixture was stirred at room temperature for 5 min. Finally, the hydrogenation bottle was put into the autoclave, the hydrogen was replaced three times and then filled with 30 bar H2, and reacted at 50°C for 16h. After the reaction is completed, the hydrogen is released carefully, the solvent is spin-dried under reduced pressure, and purified by silica gel column to obtain the hydrogenated product (R)-3-(dimethylamino)-1-(2-thienyl)-1-propanol, which is a pale yellow solid. >99%conversion, 97%yield, 74%ee,
References:
CN113024615,2021,A Location in patent:Paragraph 0115-0116

5424-47-5
209 suppliers
$9.00/5g

132335-49-0
34 suppliers
inquiry
13196-35-5
26 suppliers
inquiry

132335-49-0
34 suppliers
inquiry
88-15-3
597 suppliers
$5.00/10g

132335-49-0
34 suppliers
inquiry