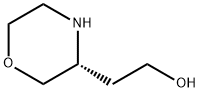
(R)-3-Hydroxyethylmorpholine synthesis
- Product Name:(R)-3-Hydroxyethylmorpholine
- CAS Number:917572-32-8
- Molecular formula:C6H13NO2
- Molecular Weight:131.17
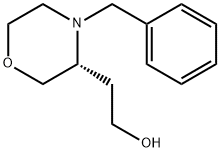
917572-31-7
1 suppliers
inquiry

917572-32-8
28 suppliers
inquiry
Yield:917572-32-8 98%
Reaction Conditions:
Stage #1: 2-[(3R)-4-benzylmorpholin-3-yl]ethanolwith hydrogen;acetic acid;palladium(II) hydroxide/carbon at 20; under 3000.3 Torr; for 17 h;
Stage #2: with ammonia in methanol;
Steps:
6.e
(e) 2-[(3R)-Morpholin-3-yl]ethanol; To a solution of 2-[(3i?)-4-benzylmorρholin-3-yl]ethanol (0.42 g, 1.9 mmol) in ethanol (10 mL) was added palladium hydroxide (20% on carbon, 0.27 g) and acetic acid (0.2 mL). The mixture was stirred under hydrogen at 4 bar and RT for 17 h. The catalyst was filtered off and the solvent was removed by evaporation. The residue was re-dissolved in ethanol (1 mL) and THF (10 mL). The solution was filtered through a cation exchange column it) (Isolute SCX-2, 10 g). The column was washed with THF and then the product was eluted with ammonia-saturated methanol. The solvent was removed by evaporation and there was obtained 0.24 g (98%) of 2- [(3Λ)-morρholin-3-yl] ethanol as an oil. 1H NMR (500 MHz, CD3OD): 1.5-1.6 (m, 2H), 2.8-3.0 (m, 3H), 3.2 (t, IH), 3.5 (m, IH)3 3.6-3.7 (t, 2H), 3.8 (m, 2H).
References:
WO2006/137790,2006,A1 Location in patent:Page/Page column 34
917572-28-2
44 suppliers
$73.00/100mg

917572-32-8
28 suppliers
inquiry

917572-29-3
22 suppliers
inquiry

917572-32-8
28 suppliers
inquiry

917572-30-6
13 suppliers
inquiry

917572-32-8
28 suppliers
inquiry
101376-26-5
93 suppliers
$23.00/100mg

917572-32-8
28 suppliers
inquiry