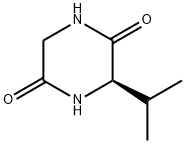
(R)-3-Isopropyl-2,5-piperazinedione synthesis
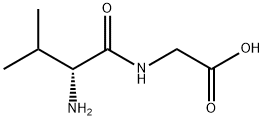
- Product Name:(R)-3-Isopropyl-2,5-piperazinedione
- CAS Number:143673-66-9
- Molecular formula:C7H12N2O2
- Molecular Weight:156.18
![Glycine, N-[(phenylmethoxy)carbonyl]-D-valyl-, methyl ester](/CAS/20210305/GIF/32402-31-6.gif)
32402-31-6
0 suppliers
inquiry

143673-66-9
116 suppliers
$9.00/250mg
Yield: 53%
Reaction Conditions:
Stage #1:N-benzyloxycarbonyl-(2R)-valinylglycine methyl ester with 5%-palladium/activated carbon;hydrogen in tetrahydrofuran at 20; under 775.743 Torr; for 2 h;
Stage #2: in toluene at 110 - 130; for 20 h;
Steps:
58
[0560] To a solution of compound 4-01-3 (40.0 g, 120 mmol) in tetrahydrofuran (100 mL) was added 5% palladium on carbon (1 g). The mixture was degassed, purged with hydrogen, and stirred at 20°C for 2 hours under hydrogen (15 psi) atmosphere. The solution was filtered through celite and concentrated under reduced pressure. The residue was dissolved in toluene (300 mL) and then stirred at 130°C for 8 hours, then stirred at 1 10°C for 12 hours. The suspension mixture was cooled to 0°C. The solid was filtered and washed with petroleum ether (500 mL *2) to afford compound 4-01-4 (11 g, 53% yield) as a white solid.
References:
CORNELL UNIVERSITY;TRI-INSTITUTIONAL THERAPEUTICS DISCOVERY INSTITUTE;LIN, Gang;NATHAN, Carl;KIRKMAN, Laura;ZHAN, Wenhu;MORGAN, Trevor;SATO, Kenjiro;HARA, Ryoma;KAWASAKI, Masanori;IMAEDA, Toshihiro;TOITA, Akinori;OKAMOTO, Rei;YUKAWA, Takafumi;ASO, Kazuyoshi;WONG, Tzu-Tshin;GINN, John, D.;FOLEY, Michael, A. WO2019/75259, 2019, A1 Location in patent:Paragraph 0560
![Glycine, N-[(1,1-dimethylethoxy)carbonyl]-D-valyl-, methyl ester](/CAS/20210305/GIF/850312-86-6.gif)
850312-86-6
1 suppliers
inquiry

143673-66-9
116 suppliers
$9.00/250mg

640-68-6
444 suppliers
$8.00/10g

143673-66-9
116 suppliers
$9.00/250mg

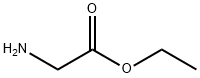
![Glycine, N-[(1,1-dimethylethoxy)carbonyl]-D-valyl-, ethyl ester](/CAS/20210305/GIF/3479-71-8.gif)