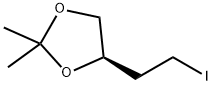
(R)-4-(2-IODO-ETHYL)-2,2-DIMETHYL-[1,3]DIOXOLANE synthesis
- Product Name:(R)-4-(2-IODO-ETHYL)-2,2-DIMETHYL-[1,3]DIOXOLANE
- CAS Number:165657-74-9
- Molecular formula:C7H13IO2
- Molecular Weight:256.08
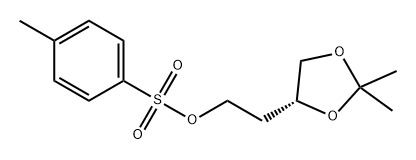
165657-75-0
0 suppliers
inquiry
![(R)-4-(2-IODO-ETHYL)-2,2-DIMETHYL-[1,3]DIOXOLANE](/CAS/GIF/165657-74-9.gif)
165657-74-9
2 suppliers
inquiry
Yield:165657-74-9 71%
Reaction Conditions:
with sodium iodide in acetone; for 2.5 h;Reflux;
Steps:
7 Compound 64
Add TEA (2 equivalents), p-toluenesulfonyl chloride (1.1) to a solution of alcohol (5 g) in 20 mL DCM in an ice/water bath.Equivalent) and DMAP (catalyst). The reaction was warmed to room temperature and stirred for 1.5 h. Concentrate the reaction mixture and pass the column colorThe spectrum (30% ethyl acetate / hexane) was purified to give 10 g of tosylate (quant.).The tosylate was dissolved in 30 mL of acetone, and sodium iodide (1.5 equivalents) was added.The reaction mixture was refluxed for 2.5 h, cooled and quenched with water.After extraction into ethyl acetate, drying, filtration, concentration and purification by column chromatography, 6.2 g of iodide 64 (from tosylate, 71% yield) was obtained.
References:
CN108368023,2018,A Location in patent:Paragraph 0094; 0096; 0097; 0178; 0179