
(R)-4-Benzyl-5-hydroxymethylmorpholin-3-one synthesis
- Product Name:(R)-4-Benzyl-5-hydroxymethylmorpholin-3-one
- CAS Number:1266238-73-6
- Molecular formula:C12H15NO3
- Molecular Weight:221.25

1235181-00-6
45 suppliers
$16.00/250mg

1266238-73-6
11 suppliers
inquiry
Yield:1266238-73-6 84%
Reaction Conditions:
with lithium tetrahydridoborate in tetrahydrofuran at 0 - 20; for 7 h;Inert atmosphere;
Steps:
(R)-4-benzyl-5-(hydroxymethyl)morpholin-3-one (21):
To a solution of the ester 20 (4.99 g, 20.0 mmol, 1.0 eq.) in THF (70 mL) was added dropwise a solution of LiBH4 (1.74 g, 80.0 mmol, 4.0 eq.) in (50 mL) at 0°C. The mixture was stirred at r.t during 7h. The solution was diluted with EtOAc (100 mL) and quenched by the addition of EtOH (10 mL), MeOH (10 mL), deionized water (20 mL) and finally saturated aqueous solution of ammonium chloride (80 mL). The layers were separated and the aqueous layers were extracted with EtOAc (3 x 50 mL). The combined organic layers were washed with brine (50 mL), dried over anhydrous Na2SO4, filtered and evaporated to dryness. The crude product was purified by column chromatography (SiO2, CyH/EtOAc 4:6) to yield the alcohol 21 (3.74 g, 16.9 mmol, 84%) as a colorless oil. 1H NMR (CDCl3, 400 MHz): δ = 7.39-7.21 (m, 5H), 5.30 (d, J = 15.0 Hz, 1H), 4.30 (d, J = 16.8 Hz, 1H), 4.21 (d, J = 16.8 Hz, 1H, 4.14-4.06 (m, 2H, H 3), 3.85 (dd, J = 11.1 Hz, J = 7.2 Hz, 1H), 3.76 (dd, J = 11.1 Hz, J = 3.8 Hz, 1H), 3.68 (dd, J = 12.0 Hz, J = 3.1 Hz, 1H), 3.30-3.21 (m, 1H). 13C NMR (CDCl3, 101 MHz): δ = 167.6, 136.6, 129.0, 128.1, 127.9, 68.1, 65.8, 61.0, 55.4, 47.8. MS (MALDI): m/z = 222.2 [M+H]+.
References:
Stojiljkovic, Uros;Meyer, Claudio;Boulay, Pierre;Hebeisen, Paul;Rageot, Denise;Wymann, Matthias P.;Borsari, Chiara [Synthesis,2023,vol. 55,# 3,p. 499 - 509] Location in patent:supporting information
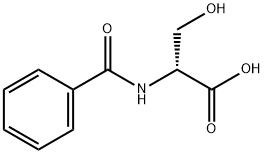
86808-09-5
1 suppliers
inquiry

1266238-73-6
11 suppliers
inquiry
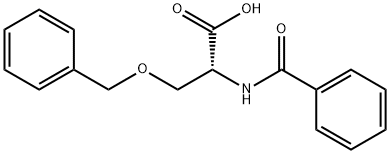
1266238-70-3
0 suppliers
inquiry

1266238-73-6
11 suppliers
inquiry
![1-Propanol, 3-(phenylmethoxy)-2-[(phenylmethyl)amino]-, (2S)-](/CAS/20210305/GIF/1266238-71-4.gif)
1266238-71-4
1 suppliers
inquiry

1266238-73-6
11 suppliers
inquiry

98-88-4
578 suppliers
$10.00/5g

1266238-73-6
11 suppliers
inquiry