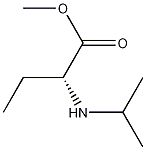
(R)-methyl 2-(isopropylamino)butanoate synthesis
- Product Name:(R)-methyl 2-(isopropylamino)butanoate
- CAS Number:947586-41-6
- Molecular formula:C8H17NO2.HCl
- Molecular Weight:195.69
Yield:947586-41-6 90%
Reaction Conditions:
Stage #1: (R)-2-aminobutyric acid;trimethyl orthoformatewith thionyl chloride in methanol at 50 - 60; for 3.5 h;Heating / reflux;
Stage #2: acetonewith sodium tris(acetoxy)borohydride in toluene at 20 - 60; for 16 h;
Stage #3: with hydrogenchloride;ammonia;watermore than 3 stages;
Steps:
ii
A suspension of 25 g (242 mmol) (R)-2-amino-butyric acid 21_ and 32 ml. (290 mmol) trimethylorthoformate in 150 mL methanol is heated to 50 °C. At this temperature 26.5 ml. (364 mmol) of thionylchloride are added in 30 minutes. Under evolution of gas the temperature increases to 60 °C. The reaction mixture is refluxed for 3 hours. 125 mL methanol are destilled off and 100 mL toluene are added. 75 mL of solvent are removed by distillation. A suspension of 77 g (364 mmol) sodium triacetoxyborohydride in 175 mL toluene is added to the reaction mixture at 60 °C. 22 mL acetone are added at 40 °C. The reaction mixture is stirred for 16 hours at room temperature. Under cooling 73 mL ammonia (25%) is added. After addition of 50 mL of demineralised water the mixture is heated to 50°C. The organic phase is separated and washed with demineralised water. 24 mL of a 10 molar solution of hydrogenchloride in ethanol is added. 125 mL of solvent are removed by distillation. 175 mL tetrahydrofurane is added and the suspension is cooled to 2 °C. The suspension is suction filtered and washed with cold tetrahydrofurane. After drying in a vacuum drying oven at 50°C, 42.9 g (90% of theory) of product 22 as hydrochloride is obtained.
References:
WO2007/90844,2007,A1 Location in patent:Page/Page column 12
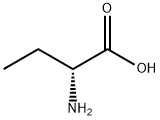
2623-91-8
370 suppliers
$5.00/5g

67-64-1
6 suppliers
$19.10/10ml
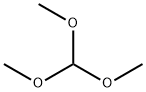
149-73-5
520 suppliers
$12.00/5g

947586-41-6
16 suppliers
inquiry
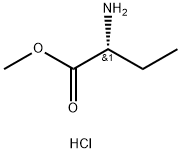
85774-09-0
77 suppliers
$5.00/250mg

67-64-1
6 suppliers
$19.10/10ml

947586-41-6
16 suppliers
inquiry