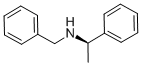
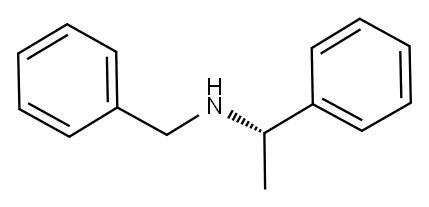
(R)-(+)-N-Benzyl-1-phenylethylamine synthesis
- Product Name:(R)-(+)-N-Benzyl-1-phenylethylamine
- CAS Number:38235-77-7
- Molecular formula:C15H17N
- Molecular Weight:211.3
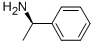
3886-69-9
557 suppliers
$14.00/25G
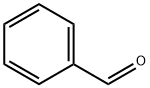
100-52-7
946 suppliers
$17.30/2g

38235-77-7
253 suppliers
$8.00/5g
Yield:38235-77-7 92%
Reaction Conditions:
Stage #1:(R)-1-phenyl-ethyl-amine;benzaldehyde in toluene for 3 h;Reflux;
Stage #2: with methanol;sodium tetrahydroborate at 0; for 2.5 h;
Steps:
4.2. (R)-N-Benzyl-1-phenylethanamine
To a solution of (R)-(+)-1-phenylethanamine (48.47 g, 0.40 mol) in toluene (300 mL), was added benzaldehyde (43.51 g, 0.41 mol). The resulting solution was heated at reflux. Stirring was then continued at reflux for around 3 h and water was removed by azeotropic distillation with a Dean-Stark trap during refluxing. After the reaction was complete, the solvent was removed by vacuum distillation to give a residue as pale yellow oil, which was then dissolved in anhydrous methanol (300 mL). The solution was cooled down to 0 °C with an ice bath, and the powdered NaBH4 (15.52 g, 0.41 mol) was added in portions over 0.5 h. After the addition was complete, the mixture was stirred at 0 °C for a further 2 h, and the reaction was traced by TLC (EtOAc/hexane = 1:6). When the reaction was complete, methanol was removed by vacuum distillation. The solid residue was then partitioned between toluene (300 mL) and water (150 mL). Two phases were separated, and the aqueous phase was extracted twice with toluene (2 × 75 mL). The extracts were combined and dried over anhydrous MgSO4. Evaporation of toluene under vacuum gave an oily residue, which was dissolved in ethanol (250 mL). Concentrated hydrochloric acid (40 mL, 12 M, 0.48 mol) was added, and the mixture was heated at reflux for 2 h. After the solution was concentrated to dryness, a residue was obtained as a pale yellow solid. A mixed solvent of ethyl acetate (100 mL) and hexane (200 mL) was added. The suspension was stirred at reflux for 2 h. After the suspension was cooled to room temperature, the off-white solid was collected on Buchner funnel by suction, and was rinsed with a mixed solvent (EtOAc/hexane = 1:3). The solid was then partitioned between ethyl acetate (400 mL) and aqueous sodium hydroxide (250 mL, 20% w/v). Two phases were separated and the aqueous phase was extracted twice with ethyl acetate (2 × 100 mL). After the combined extracts were dried over anhydrous MgSO4, evaporation of the solvent under vacuum afforded (R)-N-benzyl-1-phenylethanamine (78.1 g, 0.37 mol) as colorless oil in 92% yield. inlMMLBox (c 1.2, EtOH) {lit.63 inlMMLBox (c 1.1, EtOH)}. 1H NMR (CDCl3) δ 1.40 (d, J = 6.6 Hz, 3H), 3.63 (d, J = 13.1 Hz, 1H), 3.69 (d, J = 13.1 Hz, 1H), 3.84 (q, J = 6.6 Hz, 1H), 7.24-7.40 (m, 10H). MS m/z (%) 212 (M++1, 100), 196 (56), 91 (48).
References:
Zhu, Rui-Heng;Shi, Xiao-Xin [Tetrahedron Asymmetry,2011,vol. 22,# 4,p. 387 - 393] Location in patent:experimental part
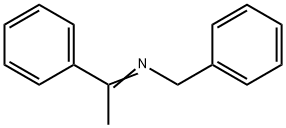
14428-98-9
1 suppliers
inquiry

38235-77-7
253 suppliers
$8.00/5g
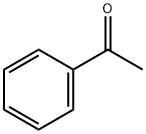
98-86-2
745 suppliers
$9.00/5g
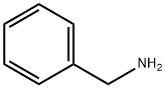
100-46-9
462 suppliers
$5.00/5 g
17480-69-2
185 suppliers
$5.00/1g

38235-77-7
253 suppliers
$8.00/5g