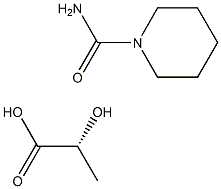
R-piperidinecarboxamide D-lactic acid synthesis
- Product Name:R-piperidinecarboxamide D-lactic acid
- CAS Number:1262398-24-2
- Molecular formula:C9H18N2O4
- Molecular Weight:218.2502
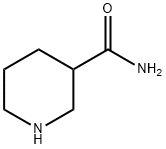
4138-26-5
203 suppliers
$11.00/5g
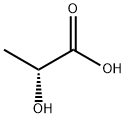
10326-41-7
243 suppliers
$6.00/25g

1262398-24-2
5 suppliers
inquiry
Yield:1262398-24-2 42.7%
Reaction Conditions:
in water;ethyl acetate;butan-1-ol at 10 - 70; for 17 h;Resolution of racemate;
Steps:
1
Ten grams (10 g, 78.0 mmol) of nipecotamide was dissolved in a mixed solvent of 30 mL of 1-butanol and 30 mL of ethyl acetate. To the solution was added 8.53 g (98.6% ee, 90% aqueous solution, 85.8 mmol) of D-lactic acid, and the solution was stirred at 70° C. To the reaction mixture was added a small amount of D-lactic acid salt of R-nipecotamide, which was prepared in advance, as a seed crystal, and a diastereomer salt was precipitated. The reaction mixture was stirred for 1 hour at the same temperature, and then was cooled to 10° C. while stirring for 6 hours. The mixture was further stirred for 10 hours. The precipitated diastereorner salt was filtered and collected. The diastereomer salt was washed with a mixed solvent of 10 mL of 1-butanol and 10 mL of ethyl acetate at approximately 5° C. After drying, the obtained white diastereomer salt weighed 7.27 g. The diastereomer salt was D-lactic acid salt of R-nipecotamide, and the yield was 42.7%. The optical purity of R-nipecotamide obtained from the diastereomer salt was analyzed and was found to be 98.0% ee.1H-NMR (DMSO-d6, 400 MHz) δppm: 7.47 (1H, s), 6.95 (1H, s), 3.71 (1H, q, J=13.7, 6.8 Hz), 3.14-3.02 (2H, m), 2.80-2.64 (2H, m), 2.54-2.44 (1H, m), 1.90-1.84 (1H, m), 1.74-1.67 (1H, m), 1.62-1.43 (2H, m), 1.14 (3H, d, J=6.8 Hz).
References:
US2012/123128,2012,A1 Location in patent:Page/Page column 3-4
98-92-0
1278 suppliers
$5.00/5g

1262398-24-2
5 suppliers
inquiry