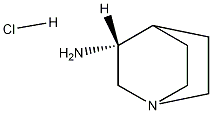
(R)-quinuclidin-3-amine hydrochloride synthesis
- Product Name:(R)-quinuclidin-3-amine hydrochloride
- CAS Number:137661-31-5
- Molecular formula:C7H15ClN2
- Molecular Weight:162.6604
![Furo[2,3-c]pyridine-5-carboxylic acid (9CI)](/CAS/GIF/478148-62-8.gif)
478148-62-8
64 suppliers
$45.00/10mg

137661-31-5
29 suppliers
$65.00/2.5mg
![N-(3R)-1-Azabicyclo[2.2.2]oct-3-yl-furo[2,3-
c]pyridine- 5-carboxamide
hydrochloride PHA 543613
hydrochloride](/CAS/20150408/GIF/478149-53-0.gif)
478149-53-0
19 suppliers
$151.00/5mg
Yield:478149-53-0 66%
Reaction Conditions:
with O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate;N-ethyl-N,N-diisopropylamine in chloroform at 20; for 16 h;
Steps:
9 Synthesis of N-(1-Azabicyclo[2.2.2]oct-3(R)-yl)furo[2,3-c]pyridine-5-carboxamide
First Step
Synthesis of N-(1-Azabicyclo[2.2.2]oct-3(R)-yl)furo[2,3-c]pyridine-5-carboxamide
To a solution (10 mL) of furo[2,3-c]pyridine-5-carboxylic acid (0.16 g, 1.0 mmol) in chloroform, o-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate (0.57 g, 1.5 mmol), diisopropylethylamine (0.70 mL, 4.0 mmol), and (R)-quinuclidine-3-amine hydrochloride (0.20 g, 1.0 mmol) were added, and the resulting mixture was stirred at room temperature.
Sixteen hours later, distilled water was added thereto, and the resultant was then extracted with chloroform.
The organic layer was washed with brine, then dried over anhydrous sodium sulfate and concentrated.
The obtained crude product was purified by silica gel column chromatography (amine silica gel DM1020, Fuji Silysia Chemical Ltd., chloroform alone to chloroform/methanol=98/2) to obtain the title compound (0.18 mg; 66%) as a colorless liquid.
1H-NMR (400 MHz, CDCl3)
δ: 1.40-1.55 (1H, m), 1.65-1.74 (2H, m), 1.80-1.90 (1H, m), 2.02-2.06 (1H, m), 2.63-2.70 (1H, m), 2.75-3.00 (4H, m), 3.39-3.46 (1H, m), 4.12-4.20 (1H, m), 6.89-6.92 (1H, m), 7.80 (1H, d, J=2.0 Hz) 8.25-8.35 (1H, m), 8.46-8.49 (1H, m), 8.75-8.78 (1H, m).MS (ESI) [M+H]+272
References:
US2014/128606,2014,A1 Location in patent:Paragraph 0190; 0191; 0192; 0193; 0194; 0195
![6-Nitrobenzo[b]thiophene-2-carboxylic acid](/CAS/20150408/GIF/19983-42-7.gif)
19983-42-7
31 suppliers
inquiry

137661-31-5
29 suppliers
$65.00/2.5mg
![Benzo[b]thiophene-2-carboxamide, N-(3R)-1-azabicyclo[2.2.2]oct-3-yl-6-nitro-, hydrochloride (1:1)](/CAS/20211123/GIF/550998-69-1.gif)
550998-69-1
0 suppliers
inquiry