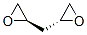
(R,R)-1,2,4,5-Diepoxypentane synthesis
- Product Name:(R,R)-1,2,4,5-Diepoxypentane
- CAS Number:109905-51-3
- Molecular formula:C5H8O2
- Molecular Weight:100.1158
136030-29-0
0 suppliers
inquiry
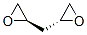
109905-51-3
2 suppliers
inquiry
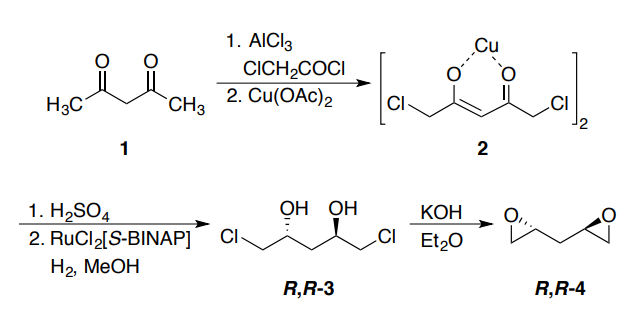
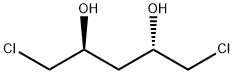
Yield:>97 % ee
Reaction Conditions:
with potassium hydroxide in diethyl ether at 0 - 25; for 3 h;
References:
Rychnovsky, Scott D.;Griesgraber, George;Powers, Jay P. [Organic Syntheses,2000,vol. 77,p. 1 - 1]

92691-36-6
0 suppliers
inquiry
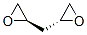
109905-51-3
2 suppliers
inquiry
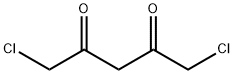
40630-12-4
17 suppliers
inquiry
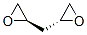
109905-51-3
2 suppliers
inquiry
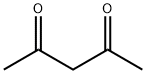
123-54-6
578 suppliers
$10.00/25ml
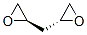
109905-51-3
2 suppliers
inquiry

591-93-5
45 suppliers
$61.29/1g
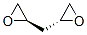
109905-51-3
2 suppliers
inquiry