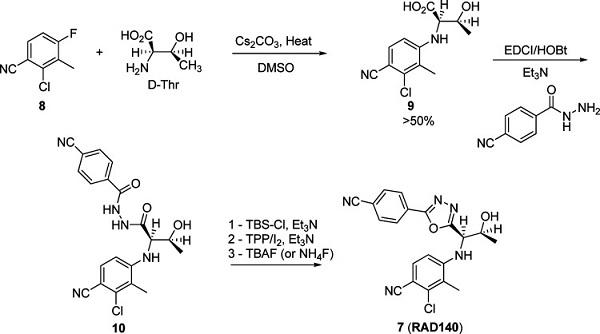
RAD140 synthesis
- Product Name:RAD140
- CAS Number:1182367-47-0
- Molecular formula:C20H16ClN5O2
- Molecular Weight:393.83
![Benzonitrile, 2-chloro-4-[[(1R,2S)-1-[5-(4-cyanophenyl)-1,3,4-oxadiazol-2-yl]-2-[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]amino]-3-methyl-](/CAS/20210305/GIF/1182367-50-5.gif)
1182367-50-5
1 suppliers
inquiry

1182367-47-0
182 suppliers
$50.00/500μg
Yield:1182367-47-0 68%
Reaction Conditions:
with tetrabutyl ammonium fluoride in tetrahydrofuran at -40 - 20; for 4 h;Inert atmosphere;
Steps:
22
Example 22; 2-chloro-4-((1R,2S)-1-(5-(4-cyanophenyl)-1,3,4-oxadiazol-2-yl)-2-hydroxypropylamino)-3-methylbenzonitrile; To a 25 mL round bottom flask equipped with a magnetic stirrer and a septum was added 4-((1R,2S)-2-(tert-butyldimethylsilyloxy)-1-(5-(4-cyanophenyl)-1,3,4-oxadiazol-2-yl)propylamino)-2-chloro-3-methylbenzonitrile (9.98 g, 19.6 mmol), followed by anhydrous THF (245 mL) under an atmosphere of nitrogen. The reaction mixture was cooled to -40° C. via a CO2/acetone bath. To this was added tetrabutylammonium fluoride as a 1M solution in THF (22.54 mL, 22.54 mmol) producing an instant color change to yellow. This was allowed to warm to room temperature for a period of 4 h. The solvent was then removed under reduced pressure to yield a black residue. This residue was taken up in EtOAc (200 mL) and washed with water (2×250 mL), brine (1×200 mL) and dried over Na2SO4. The solution was then filtered and concentrated under reduced pressure using rotary evaporation to reveal a yellow solid. This was purified via silica gel chromatography eluted with hexanes-EtOAc (20:80) the title compound as a white solid (5.23 g, 68%). 1H NMR (500 MHz, CDCl3, δ in ppm) 8.10 (d, J=8.4 Hz, 2H), 7.80 (d, J=8.4 Hz, 2H), 7.40 (d, J=8.4 Hz, 1H), 6.65 (d, J=8.4 Hz, 1H), 5.27 (d, J=8.0 Hz, 1H), 4.80 (dd, J=2.8, 8.4 Hz, 1H), 4.62-4.65 (m, 1H), 2.91 (br s, 1H), 2.37 (s, 3H), 1.46 (d, J=6.4 Hz, 3H).
References:
US2009/253758,2009,A1 Location in patent:Page/Page column 56