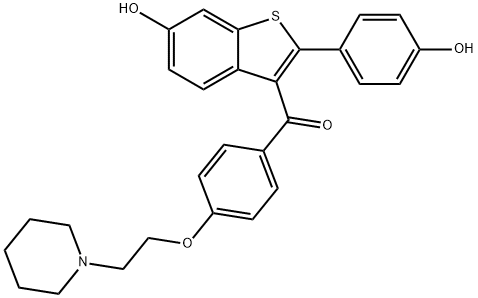
Raloxifene synthesis
- Product Name:Raloxifene
- CAS Number:84449-90-1
- Molecular formula:C28H27NO4S
- Molecular Weight:473.58

82640-04-8
445 suppliers
$25.00/1g

84449-90-1
179 suppliers
inquiry
Yield:84449-90-1 100%
Reaction Conditions:
Stage #1: raloxifene hydrochloridewith sodium hydroxide in water;acetone at 20;
Stage #2: with acetic acid in water;acetone at 16 - 20; pH=8; for 1.75 h;
Steps:
6.6.1.a
a) Preparation from 1-(2-{4-[6-hydroxy-2-(4-hydroxy-phenyl)-benzo[b]thiophene-3- carbonyl]-phenoxy}-ethyl)-piperidinium chloride 0.37 methanol 0.51 methylene chloride using acetone as antisolvent:98.0 g of 1-(2-{4-[6-hydroxy-2-(4-hydroxy-phenyl)-benzo[b]thiophene-3-carbonyl]-phenoxy}- ethyl)-piperidinium chloride 0.37 methanol · 0.51 methylene chloride (88.49 g, 173.5 mmol, calcd. dry) prepared according to example 7 item b were treated with aq. sodium hydroxide (2 N, 415 mL, 830 mmol) and acetone (580 mL) at ambient temperature with stirring. Acetic acid (aq., 2 N, 330 mL, 660 mmol) was then added slowly (approx. 0.75 h) at 16 - 18 °C to adjust a pH of 8. After seeding, the resulting suspension was stirred for 1 h at room temperature °C and filtered. The solid was washed with water (10 x 50 ml_).A sample (1.0 g) was dried at 60 °C under reduced pressure to yield 0.76 g of [6-Hydroxy-2-(4- hydroxy-phenyl)-benzo[b]thiophen-3-yl]-[4-(2-piperidin-1-yl-ethoxy)-phenyl]-methanone · 0.98 H20 · 0.36 acetone. Yield: quantitative. Purity: 99.6 % (HPLC). H20: 3.46 % by weight, corresponding to approx. 0.98 molar equivalents (KFT). Aceton: 4.03 % by weight,corresponding to approx. 0.36 molar equivalents (GC). Mp.: 126.7 - 129.8 °C. DSC: Peak at 1 19.89 °C. 1H NMR (300 MHz, DMSO-d6, ppm): δ 9.75 (b, 2 H, -OH), 7.65 (d, 2 H, arom ), 7.34 (d, 1 H, arom.), 7.25 (d, 1 H, arom.), 7.18 (d, 2 H, arom.), 6.91 (d, 2 H, arom.), 6.86 (dd, 1 H, arom.), 6.68 (d, 2 H, arom.), 4.06 (t, 2 H, -OCH2CH2NR2), 2.60 (t, 2 H, -OCH2CH2NR2), 2.37 (m, 4 H, piperidine), 1.60 - 1.22 (m, 6 H, piperidine). 13C-{H}-NMR (75 MHz, DMSO-d6, ppm): δ 192.50 (>C=0), 162.78 (=C<), 157.80 (=C<), 155.39 (=C<), 140.26 (=C<), 139.19 (=C<), 132.26 (=C<), 131.71 (=CH-), 129.73 (=C<), 129.65 (=C<), 129.62 (=CH-), 123.75 (=C<), 123.25 (=CH-), 1 15.64 (=CH-), 1 15.14 (=CH-), 114.45 (=CH-), 107.07 (=CH-), 65.96 (-CH2-), 57.05 (-CH2-), 54.27 (-CH2-), 25.49 (-CH2-), 23.83 (-CH2-). IR (KBr, cm"1): 3194, 2935, 1715, 1627, 1592, 1543, 1500, 1469, 1350, 1314, 1259, 1 170, 1129, 1033, 1024, 943, 909, 836, 791 , 764, 646, 637, 534. LC/MS (ESI(+), RT; 10.18 min): m/z = 474.0 [MH-H20]+.
References:
WO2011/47878,2011,A2 Location in patent:Page/Page column 43-44
![(4-(2-(PIPERIDIN-1-YL)ETHOXY)PHENYL)(6-METHOXY-2-(4-METHOXYPHENYL)BENZO[B]THIOPHEN-3-YL)METHANONE](/CAS/GIF/84541-38-8.gif)
84541-38-8
27 suppliers
$130.00/10mg

84449-90-1
179 suppliers
inquiry
![Methanone, [6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thien-3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]-, hydrobromide (1:1)](/CAS/20211123/GIF/1293408-87-3.gif)
1293408-87-3
0 suppliers
inquiry

84449-90-1
179 suppliers
inquiry
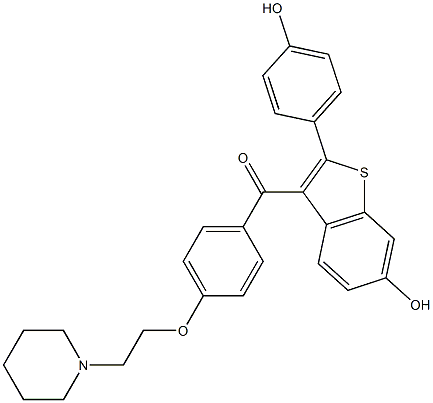
84449-85-4
14 suppliers
inquiry

84449-90-1
179 suppliers
inquiry
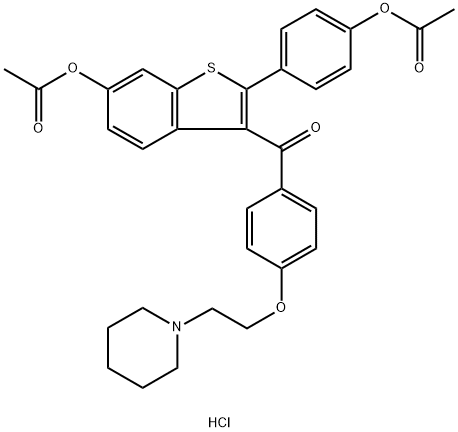
84449-82-1
2 suppliers
inquiry

84449-90-1
179 suppliers
inquiry