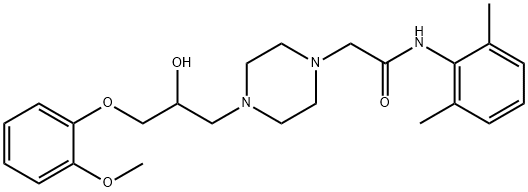
Ranolazine synthesis
- Product Name:Ranolazine
- CAS Number:95635-55-5
- Molecular formula:C24H33N3O4
- Molecular Weight:427.54
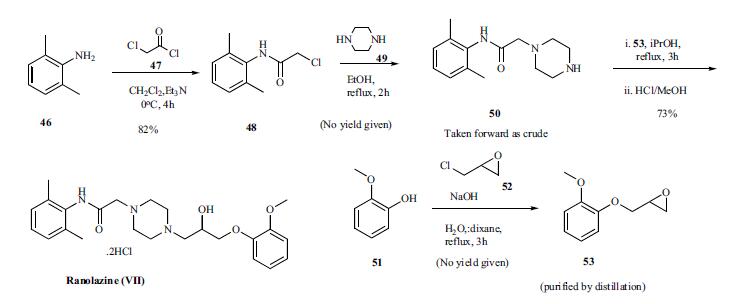
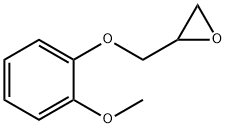
2210-74-4
299 suppliers
$6.00/250mg

5294-61-1
281 suppliers
$28.00/5g

95635-55-5
366 suppliers
$5.00/100mg
Yield:95635-55-5 85%
Reaction Conditions:
with Sulfated tungstate at 70; for 2 h;Green chemistry;
Steps:
General procedure for epoxide opening reaction
General procedure: Sulfated tungstate (10 wt%) was added to a solution of cyclohexene oxide (1 g, 10.18 mmol) and aniline (0.95 g, 10.18 mmol) in solvent-free condition and the mixture was stirred at room temperature. The progress of the reaction was monitored by TLC. After the completion of the reaction, the reaction mixture was diluted with ethyl acetate (15 ml) and filtered to recover the catalyst. Organic layer washed with water (10 ml), dried over Na2SO4 and concentrated under reduced pressure to give the crude product which was purified by chromatography on silica gel (60-120) with hexane-ethyl acetate (8:2) as eluent to get pure 2-(phenylamino) cyclohexanol as a white solid.
References:
Pathare, Sagar P.;Akamanchi, Krishnacharya G. [Tetrahedron Letters,2013,vol. 54,# 48,p. 6455 - 6459]
![1-Piperazineethanol, a-[(2-methoxyphenoxy)methyl]-](/CAS/GIF/162712-35-8.gif)
162712-35-8
27 suppliers
$378.00/1g
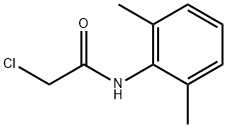
1131-01-7
358 suppliers
$7.00/5g

95635-55-5
366 suppliers
$5.00/100mg
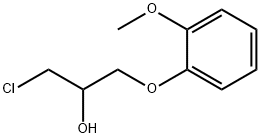
25772-81-0
12 suppliers
inquiry

5294-61-1
281 suppliers
$28.00/5g

95635-55-5
366 suppliers
$5.00/100mg
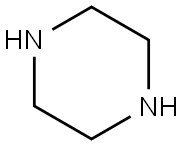
110-85-0
550 suppliers
$5.00/5G

2210-74-4
299 suppliers
$6.00/250mg

1131-01-7
358 suppliers
$7.00/5g

95635-55-5
366 suppliers
$5.00/100mg
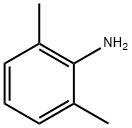
87-62-7
453 suppliers
$10.00/5G

95635-55-5
366 suppliers
$5.00/100mg