
RARECHEM AL BD 0561 synthesis
- Product Name:RARECHEM AL BD 0561
- CAS Number:40016-25-9
- Molecular formula:C15H24O
- Molecular Weight:220.35
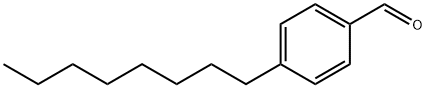
49763-66-8
67 suppliers
$90.00/500mg

40016-25-9
13 suppliers
$198.00/1g
Yield:40016-25-9 100%
Reaction Conditions:
Stage #1: 4-octylbenzaldehydewith methanol;sodium tetrahydroborate at 20; for 0.5 h;
Stage #2: with water;sodium hydroxide in diethyl ether;
Steps:
6.A
NaBH4 (0.04 g; 1 .06 mmol) was added portion wise to a solution of the product of Example 3, Step A in MeOH (5 ml) at room temperature, with vigorous stirring. After 30 min of stirring, the mixture was evaporated to dryness, diluted to 10 ml with Et2O and washed with 1 N NaOH, H2O, brine, dried over anhydrous MgSO4 and filtered. The filtrate was evaporated under reduced pressure to give the title compound (0.082 g; 100%), as colourless syrup, which was used in next step without further purification. 1 H-NMR (CDCI3) 7.26 (d, 2H, J = 8 Hz); 7.15 (d, 2H, J = 8 Hz); 4.64 (s, 2H); 2.58 (t, 2H, J = 7.9 Hz); 1 .56 (m, 3H); 1 .26 (m, 10H); 0.86 (t, 3H, J = 6.9 Hz).
References:
WO2010/43000,2010,A1 Location in patent:Page/Page column 51

54256-51-8
19 suppliers
$80.00/1g

40016-25-9
13 suppliers
$198.00/1g

629-27-6
149 suppliers
$5.00/5g
18282-51-4
206 suppliers
$10.00/1g

40016-25-9
13 suppliers
$198.00/1g
7440-44-0
298 suppliers
$12.00/25g

40016-26-0
10 suppliers
$48.29/250mg

40016-25-9
13 suppliers
$198.00/1g
99209-58-2
0 suppliers
inquiry

40016-25-9
13 suppliers
$198.00/1g