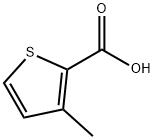
RARECHEM AL BI 0178 synthesis
- Product Name:RARECHEM AL BI 0178
- CAS Number:14300-64-2
- Molecular formula:C8H10O2S
- Molecular Weight:170.23
Yield:14300-64-2 99%
Reaction Conditions:
with thionyl chloride for 16 h;Heating / reflux;
Steps:
15.A
A. To a stirred solution of 3-methylthiophene-2-carboxylic acid (5.00 g, 35.17 mmol) in ethanol (50 mL) was added thionyl chloride (2.60 mL, 35.62 mmol). The resulting reaction mixture was stirred at reflux for 16 h, and allowed to cool to ambient temperature and concentrated in vacuo. The residue was dissolved in ethyl acetate (200 mL) and washed with saturated aqueous sodium bicarbonate solution (3 x 100 mL) and brine (100 mL). The organic layer was dried over sodium sulfate, filtered and
References:
WO2008/74835,2008,A1 Location in patent:Page/Page column 79-80