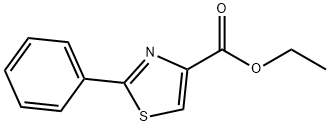
RARECHEM AL BI 1318 synthesis
- Product Name:RARECHEM AL BI 1318
- CAS Number:59937-01-8
- Molecular formula:C12H11NO2S
- Molecular Weight:233.29

70-23-5
310 suppliers
$6.00/5g
2227-79-4
222 suppliers
$6.00/5g

59937-01-8
64 suppliers
$43.00/250mg
Yield:59937-01-8 94%
Reaction Conditions:
in ethanol at 20; for 2 h;Reflux;
Steps:
85A Ethyl 2-phenylthiazole-4-carboxylate
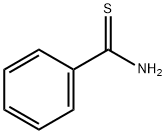
A solution of benzothioamide (3.00 g, 21.87 mmol) in EtOH (70 mL) was treated dropwise with ethyl bromopyruvate (5.10 g, 26.2 mmol) and stirred at room temperature for 30 min before being heated at reflux for 1.5 h. The cooled mixture was diluted with ethyl acetate (200 mL), washed (aqueous NaHC03, brine), dried over anhydrous MgSO4 and evaporated. The residue was purified on the ISCO using a REDISEP 80 g column (10 to 20% EtOAc-hexane) to give the title compound (4.82 g, 94%) as a yellow oil. LCMS (APCI): calcd for C12H12NO2S [M+H]+ m/z 234.05, found 234.1. 1H NMR (CDCl3, 400 MHz) δ ppm: 8.14 - 8.19 (m, 1H), 7.98 - 8.07 (m, 2H), 7.41 - 7.51 (m, 3H), 4.46 (q, J= 7.2 Hz, 2H), 1.44 (t, J= 7.2 Hz, 3H)
References:
WO2013/163279,2013,A1 Location in patent:Paragraph 00226
89530-19-8
0 suppliers
inquiry

59937-01-8
64 suppliers
$43.00/250mg
100367-77-9
256 suppliers
$5.00/250mg
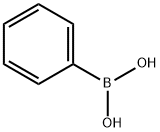
98-80-6
720 suppliers
$5.00/5g

59937-01-8
64 suppliers
$43.00/250mg

425392-44-5
95 suppliers
$59.00/250mg

98-80-6
720 suppliers
$5.00/5g

59937-01-8
64 suppliers
$43.00/250mg

425392-45-6
44 suppliers
$7.00/100mg

425392-46-7
0 suppliers
inquiry
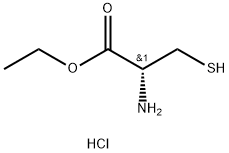
868-59-7
283 suppliers
$10.00/1g
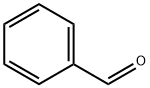
100-52-7
941 suppliers
$20.37/250g

59937-01-8
64 suppliers
$43.00/250mg