
RARECHEM AN KA 0422 synthesis
- Product Name:RARECHEM AN KA 0422
- CAS Number:104037-38-9
- Molecular formula:C11H12N2
- Molecular Weight:172.23

14068-28-1
22 suppliers
inquiry

104037-38-9
42 suppliers
$45.00/25mg
Yield: 35%
Reaction Conditions:
with ammonia;hydrogen in ethanol;water under 1810.07 Torr;
Steps:
a2.3 a2.3) 2-Quinolin-2-yl-ethanamine
a2.3) 2-Quinolin-2-yl-ethanamine A solution of 2-(cyanomethyl)quinoline (50 g, 2971 mmol, see Example a2.2)) in ethanol (400 ml) was added Raney nickel (1.7 g) and aqueous ammonia (concentrated, 250 ml). The mixture was hydrogenated with hydrogen gas (pressure: 35psi) overnight. The mixture was filtered and concentrated. The residue was dissolved in water (500 ml). and the pH value was adjusted to 1 with concentrated aqueous HC1 solution. The mixture was then extracted with ethyl acetate (3x400 ml ) to remove impurities. The pH value of the aqueous layer was adjusted to 6-8 by adding aqueous sodium hydroxide. Afterwards the aqueous layer was extracted with DCM (3x300 ml) to remove impurities. The aqueous layer was then concentrated. DCM (200 ml) and MeOH (50 ml) were added to the solid, the mixture was filtered through a Buchner funnel. The solution was concentrated to dryness to afford the title compound 6 (18 g, 35%> yield). LC-MS (ESI+): m/e 173 (M+H)+, Rt: 0.95 min; 1H-NMR (400 MHz, DMSO- 6) : δ = 3.32 (s, 4H); 7.50 (d, J=8.4Hz, 1H); 7.59 (t, J=7.4Hz, 1H); 7.76 (t, J=7.6Hz, 1H); 7.97 (d, J=8Hz, 1H); 8.02 (d, J=8.8Hz, 1H); 8.27 (br, 2H); 8.34 (d, J=8.4Hz,lH).
References:
ABBVIE DEUTSCHLAND GMBH & CO. KG;ABBVIE INC.;GENESTE, Hervé;OCHSE, Michael;DRESCHER, Karla;BEHL, Berthold;LAPLANCHE, Loic;DINGES, Jürgen;JAKOB, Clarissa WO2014/140184, 2014, A1 Location in patent:Page/Page column 73
![1-[2-(2-quinolinyl)ethyl]-2,5-pyrrolidinedione](/CAS/20180703/GIF/74273-98-6.gif)
74273-98-6
1 suppliers
inquiry

104037-38-9
42 suppliers
$45.00/25mg
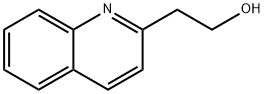
1011-50-3
72 suppliers
$58.00/100mg

104037-38-9
42 suppliers
$45.00/25mg
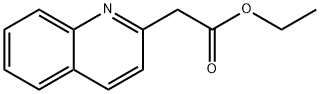
5100-57-2
33 suppliers
$180.00/50mg

104037-38-9
42 suppliers
$45.00/25mg
![2-[2-[(E)-2-[4-(dimethylamino)phenyl]ethenyl]-6-methylpyran-4-ylidene]propanedinitrile](/CAS/20180601/GIF/96042-30-7.gif)