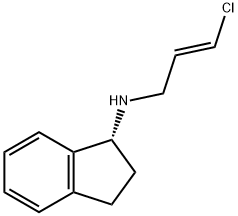
Rasagiline Impurity 4 synthesis
- Product Name:Rasagiline Impurity 4
- CAS Number:1175018-79-7
- Molecular formula:C12H14ClN
- Molecular Weight:207.7
136236-51-6
156 suppliers
$5.00/1mg
1175018-74-2
22 suppliers
inquiry

1175018-79-7
14 suppliers
inquiry
1175018-73-1
17 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: rasagilinewith hydrogenchloride in water; for 33 h;Reflux;
Stage #2: with sodium hydroxide in water; pH=14;
Steps:
1.1; 1.2
1. (R)-PAI base (30 g) and 32% HCl (100 ml) were refluxed for 33 hours (see scheme 2). The reaction mixture was cooled, toluene (150 ml) and water (150 ml) were added and the mixture was stirred for 15 min. The aqueous phase was separated, brought to pH 14 with 47% NaOH and extracted with 150 ml toluene. The organic phase was evaporated to give 36 g dark brown oil which exhibited 3 spots in TLC, two of which were the desired compounds (hexane-4/EA-6,2-Chloro-AAI base: Rf=0.67; cis-3-chloro-AAI base: Rf=0.33, visualized in UV). The third spot had the same Rf as the starting material (0.47) and it was thus thought to be the starting material. However, when trans-3-chloro-AAI (see below) was synthesized it was determined that both the trans and the starting material have the same Rf values under the TLC conditions used. Subsequent testing revealed that third spot was due mostly to PAI and only trace amounts of the trans isomer were formed. 2. The compounds formed in step 1 were separated by filtering-column chromatography [Aldrichimica Acta, 21(4), 106-107 (1998)]. Thus 15 g of the product of step 1 was separated on 400 g silica (Merck type 9385) with a mixture of hexane-7/EA-3 to give 5.6 g pure (R)-2-chloro-AAI (15% overall yield) and 5.0 g cis-3-chloro-AAI base (13% overall yield for steps 1 and 2). 3. To 6.7 g of 2-chloro-AAI base in 100 ml diethyl ether was added 9 ml of 14.5% HCl/ethanol solution. The reaction mixture was stirred for ? hr at ambient temperature and ? hr at 5-10° C. The precipitate was filtered, washed with ether and dried in vacuum at 50° C. to give 7.1 g (90% yield) of the pure compound, m.p.=166-169° C. (see scheme 2). The compound exhibited satisfactory NMR and IR spectra which confirmed its structure. 1H-NMR Spectrum The 1H-NMR spectrum is shown in FIG. 1. NMR Peak assignments are listed below: Proton δ (ppm) Multiplicity Coupling Constant (J, Hz) H1 (1H) 4.80 dd 7, 5 H2 (2H) 2.33 m -H3I (1H) 2.82 ddd 17, 8, 6H3II (1H) 3.18 dt 17, 8 H5, H6, H7 (3H) 7.24-7.25 m - H8 (1H) 7.85 d 15 H10 (2H) 3.83, 3.96 AB q 15H12I (1H) 5.65 s -H12II (1H) 5.95 s - H13 (2H) 10.10 broad s - Mass Spectroscopy (MS) The mass spectrum of 2-chloro-AAI-HCl was obtained using a Finnigan 4000 Quadropole Low Resolution Mass Spectrometer, operated in electron impact (EI) mode. The electron impact (EI) spectrum exhibits molecular ions at m/z 207 [M]+. and 206 [M+.-H]+., and characteristic fragments at m/z 171 [M+.-HCl]+., 132 [M+.-C3H4Cl.]+ and 116 [M+.-C3H6ClN.]+. The EI spectrum is in agreement with the molecular formula of 2-chloro-AAI.Elemental Analysis Calculated C: 59.03% H: 6.19% N: 5.74% Found C: 58.93% H: 6.20% N: 5.68% These results correspond to the molecular formula.FT-IR SpectrumThe infrared (FT-IR) spectrum of 2-chloro-AAI-HCl is shown in FIG. 2.
References:
US7572834,2009,B1 Location in patent:Page/Page column 12-13