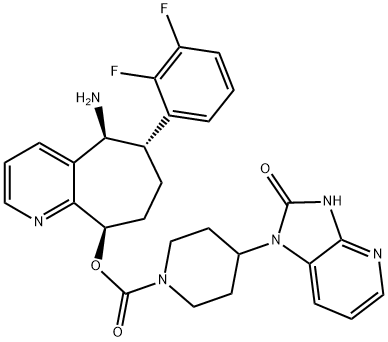
Rimegepant synthesis
- Product Name:Rimegepant
- CAS Number:1289023-67-1
- Molecular formula:C28H28F2N6O3
- Molecular Weight:534.56
![1-Piperidinecarboxylic acid, 4-(2,3-dihydro-2-oxo-1H-imidazo[4,5-b]pyridin-1-yl)-, (5S,6S,9R)-5-azido-6-(2,3-difluorophenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridin-9-yl ester](/CAS/20210111/GIF/1289023-63-7.gif)
1289023-63-7
2 suppliers
inquiry

1289023-67-1
167 suppliers
$39.00/1mg
Yield:1289023-67-1 85%
Reaction Conditions:
Stage #1: (5S,6S,9R)-5-azido-6-(2,3-difluorophenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridin-9-yl 4-(2-oxo-2,3-dihydro-1H-imidazo-[4,5-b]pyridin-1-yl)piperidine-1-carboxylatewith trimethylphosphane in tetrahydrofuran;toluene at 20; for 2 h;
Stage #2: with water in tetrahydrofuran;toluene; for 3 h;
Steps:
8
Example 8(5S, 6S,9R)-5-amino-6-(2,3-difluorophenyl)-6, 7,8,9-tetrahydro-5H- cyclohepta[b]pyridin-9-yl 4-(2-oxo-2,3-dihydro-lH-imidazo[4,5-b]pyridin-l- yl)piperidine-l -carboxylate. In a 100 mL round-bottom flask was dissolved(5S,6S,9R)-5-azido-6-(2,3-difluorophenyl)-6,7,8,9-tetrahydro-5H- cyclohepta[b]pyridin-9-yl 4-(2-oxo-2,3-dihydro-lH-imidazo[4,5-b]pyridin-l- yl)piperidine-l-carboxylate (620 mg, 1.106 mmol) (example 7) in tetrahydrofuran (5 mL) to give a colorless solution. Trimethylphosphine (3.32 mL, 3.32 mmol, 1.0 M in toluene) was added. The mixture was stirred at room temperature. After 2h, LCMS showed no starting material. Water (0.080 mL, 4.42 mmol) was added, and the mixture was stirred for another 3h. LCMS showed complete conversion to the desired product. Volatile components were removed in vacuo and the residue was directly purified by FCC upto 10% methanol in methylene chloride to afford the product (5 lOmg, 85%) as a white solid. MS(ESI)[M+H+] = 535.23; 1H NMR (400 MHz, CHLOROFORM-if) δ ppm 10.39 (br. s., 1 H) 8.52 (d, J=3.78 Hz, 1 H) 8.09 (d, J=5.04 Hz, 2 H) 7.46 (br. s., 1 H) 7.26 - 7.38 (m, 1 H) 7.06 - 7.20 (m, 3 H) 6.94 - 7.05 (m, 1 H) 6.06 - 6.23 (m, 1 H) 4.31 - 4.78 (m, 4 H) 4.05 (spt, J=6.13 Hz, 1 H) 2.57 - 3.25 (m, 3 H) 2.17 - 2.38 (m, 3 H) 1.42 - 2.04 (m, 6 H); 19F NMR (376 MHz, CHLOROFORM-if) δ ppm -136.90 (br. s., 1 F) -142.48 - -142.21 (m, 1 F).
References:
WO2011/46997,2011,A1 Location in patent:Page/Page column 45-46