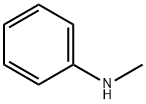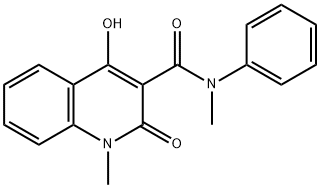
Roquinimex synthesis
- Product Name:Roquinimex
- CAS Number:84088-42-6
- Molecular formula:C18H16N2O3
- Molecular Weight:308.33
Yield: 98.6%
Reaction Conditions:
in cyclohexane for 2.5 h;Reflux;Molecular sieve;Solvent;
Steps:
1-5 Example 2
Mix 30.0 g 1,2-dihydro-4-hydroxy-1-methyl-2-oxoquinoline-3-carboxylic acid methyl ester (0.13 mol), 18.1 g N-methylaniline (0.17 mol) and 180 g cyclohexane, 1.8 g anhydrous calcium chloride were added to a 250 mL three-necked flask. The flask is connected to the inlet of the type A molecular sieve permeation gasification inorganic membrane device, stirred and heated to reflux, and refluxed for 2.5 h. During the reaction, the methanol and water mixture in the organic vapor passes through the type A molecular sieve permeation gasification inorganic membrane tube. The system is separated, and the solvent is refluxed back to the kettle for reaction. At the end of the reaction, a total of 5.1 g of methanol and water were collected. The reaction liquid was filtered at 60 °C, the filter cake was used as a catalyst (suitable for the next batch of reactions), the filtrate was cooled and crystallized and filtered, and the filter cake was dried to obtain 39.3 g of white solid, with a content of 99.6% (external standard for liquid spectrum), and a yield of 98.6% (Calculated as 1,2-dihydro-4-hydroxy-1-methyl-2-oxoquinoline-3-carboxylic acid methyl ester).
References:
Hunan Subo Biological Co., Ltd.;Yang Zidong;Hu Zhibin CN111732540, 2020, A Location in patent:Paragraph 0023-0028
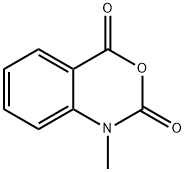
10328-92-4
141 suppliers
$18.00/1g
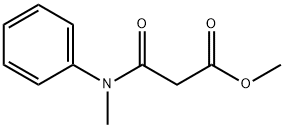
84088-88-0
1 suppliers
inquiry

84088-42-6
106 suppliers
$70.00/5mg

10328-92-4
141 suppliers
$18.00/1g

84088-42-6
106 suppliers
$70.00/5mg