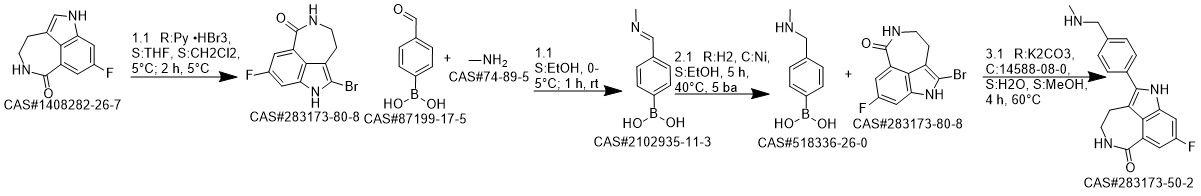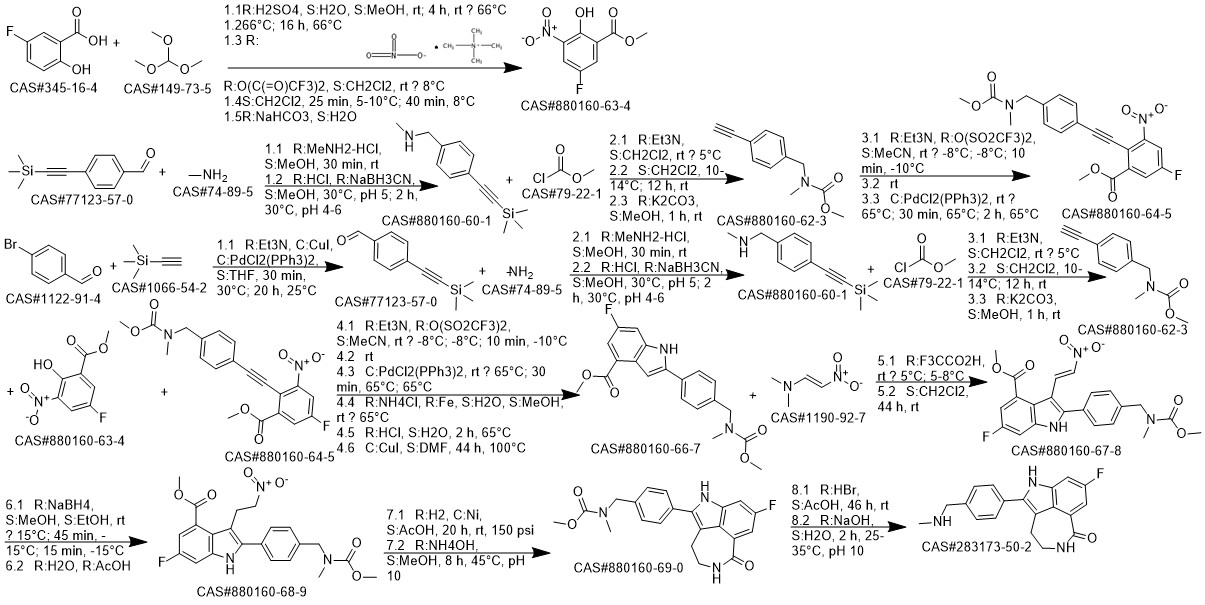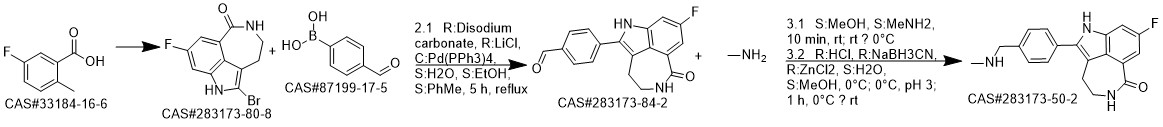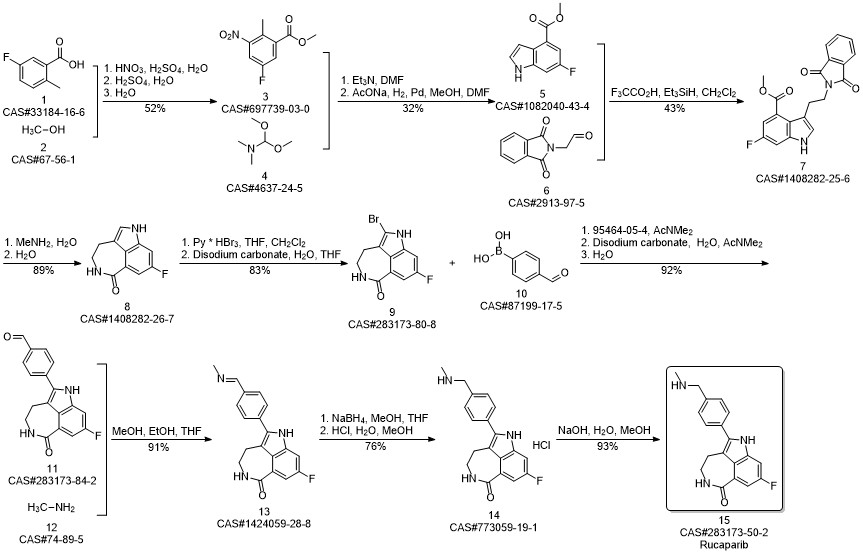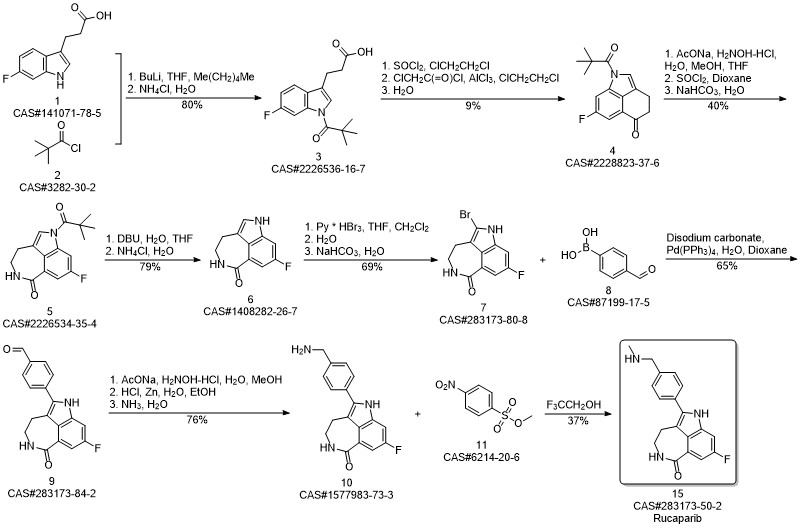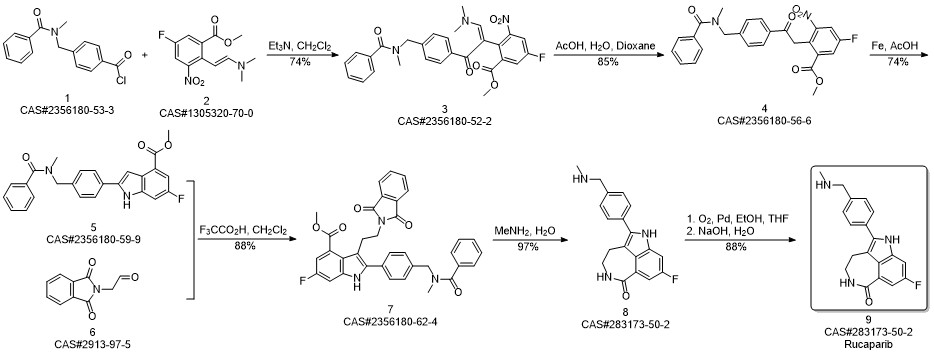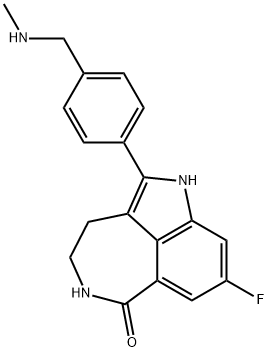
Rucaparib synthesis
- Product Name:Rucaparib
- CAS Number:283173-50-2
- Molecular formula:C19H18FN3O
- Molecular Weight:323.36
Reference: Belogi, Gianluca; Mazzoni, Andrea; Serra, Stefano; Novo, Barbara. Preparation of high-purity rucaparib via palladium-catalyzed regioselective coupling of a 4-alkoxycarbonyl-6-fluoroindole and an alkyl [(4-iodophenyl)methyl]methylcarbamate. WO 2019020508. (Olon S.p.A., Italy)
![8-fluoro-1,3,4,5-tetrahydro-azepino[5,4,3-cd]indol-6-one](/CAS/20180702/GIF/1408282-26-7.gif)
1408282-26-7
141 suppliers
$17.00/100mg
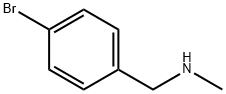
699-03-6
99 suppliers
$19.00/1g

283173-50-2
184 suppliers
$7.00/1mg
Yield:283173-50-2 71%
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);copper(II) nitrate trihydrate;caesium carbonate in N,N-dimethyl acetamide at 160; for 24 h;Inert atmosphere;regiospecific reaction;
Steps:
8-Fluoro-2-{4-[(methylamino)methyl]phenyl}-4,5-dihydro-1H-azepino[5,4,3-cd]indol-6-one (Rucaparib) (Scheme 2) [CAS Reg.No. 283173-50-2]
8-Fluoro-4,5-dihydro-1H-azepino[5,4,3-cd]indol-6-one (51.1 mg, 0.25 mmol), Cs2CO3 (162.9 mg, 0.50 mmol), Pd2dba3 (2.88 mg, 0.0032 mmol), and Cu(NO3)2·3H2O (9.08 mg, 0.075 mmol) was weighed in a glass vial under ambient atmosphere. DMA was added to obtain a total volume of 5 mL. 4-Bromo-N-methylbenzylamine (100.0 μL, 0.50mmol) was volumetrically injected into the reaction mixture. The reaction proceeded at 160 °C under magnetic stirring. After 24 h, the resulting product mixture was quenched with sat. aq NaHCO3 (50 mL), followed by extraction with EtOAc (3 × 50 mL). The crude product mixture was dried (anhyd Na2SO4) and filtered. The solvent was evaporated, and the product was purified via column chromatography (DCM-MeOH 95:5, Rf = 0.4). The silica gel column was eluted with 1 vol% of TEA prior to the separation; yield: 57.3 mg (71%); whitish solid; mp 171-173 °C; Rf = 0.4 (DCM-MeOH 95:5). IR (KBr): 3460, 3176, 3033, 2423, 1651, 1606, 1516, 1466, 1412, 1350,1209, 1105, 787, 609 cm-1. 1H NMR (400 MHz, DMSO-d6): = 2.31 (s, 3 H, CH3), 2.99 (m, 2 H,CH2), 3.46 (m, 2 H, CH2), 3.73 (s, 2 H, CH2), 7.44-7.56 (m, 5 H), 7.61 (s,1 H), 8.33 (t, J = 5.8 Hz, 1 H, CONH). 13C NMR (400 MHz, DMSO-d6): = 28.2, 36.1, 42.1, 54.9, 100.7, 100.9,110.9, 111.1, 117.0, 123.5, 124.0, 127.5, 129.9, 130.6, 136.3, 137.3,158.2, 160.4, 168.3. 19F NMR (400 MHz, DMSO-d6): = -119.9. MS (ES+): m/z (%) = 293.1 (100), 324.2 (23). HRMS: m/z [M + H]+ calcd for C19H19FN3O: 324.1512; found:324.1504.
References:
Beckers, Igor;O'Rourke, Galahad;De Vos, Dirk [Synthesis,2022,vol. 54,# 2,p. 334 - 340]