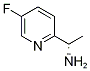
S)-1-(5-fluoropyridin-2-yl)ethanaMine synthesis
- Product Name:S)-1-(5-fluoropyridin-2-yl)ethanaMine
- CAS Number:905587-15-7
- Molecular formula:C7H9FN2
- Molecular Weight:140.16
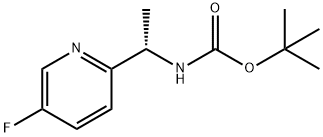
905587-16-8
11 suppliers
inquiry
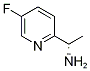
905587-15-7
34 suppliers
$55.00/10mg
Yield:905587-15-7 98%
Reaction Conditions:
Stage #1: (S)-tert-butyl-1-(5-fluoropyridin-2-yl)ethylcarbamatewith hydrogenchloride in 1,4-dioxane;dichloromethane at 20; for 3 h;
Stage #2: with sodium hydrogencarbonate in water;
Steps:
33
To the solution of (5)-tert-butyl-l-(5-fluoropyridin-2-yl)ethylcarbamate (Method 34, 12.8 g, 53.3 mmol) in DCM (100 ml) was added HCl/dioxane solution (107 ml, 4 N, 428 mmol). The reaction was stirred at room temperature for 3 hours. The solvent was removed and 50 ml of saturated sodium bicarbonate was added. The resulting aqueous solution was extracted with ether (6 x 400 ml), dried over sodium sulfate and concentrated to give the title compound (7.30 g, 98%) as pale yellow oil. 1H NMR (400 MHz) δ 8.44 (d, J= 2.8 Hz, IH), 7.66 (m, IH), 7.53 (m, IH), 4.01 (q, J= 6.8 Hz, IH), 1.94 (b, 2H), 1.26 (d, J= 6.8 Hz, 3H). MS: Calcd.: 140; Found: [M+H]+ 141.
References:
WO2006/82392,2006,A1 Location in patent:Page/Page column 104
![Carbamic acid, N-[(1S)-1-(5-fluoro-2-pyridinyl)ethyl]-, (1R,2S,5R)-5-methyl-2-(1-methylethyl)cyclohexyl ester](/CAS/20210305/GIF/915720-58-0.gif)
915720-58-0
0 suppliers
inquiry
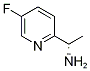
905587-15-7
34 suppliers
$55.00/10mg
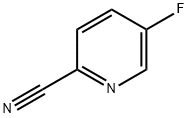
327056-62-2
257 suppliers
$5.00/1g
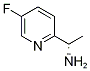
905587-15-7
34 suppliers
$55.00/10mg

905587-18-0
13 suppliers
inquiry
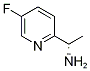
905587-15-7
34 suppliers
$55.00/10mg
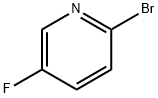
41404-58-4
400 suppliers
$5.00/1g
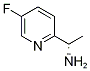
905587-15-7
34 suppliers
$55.00/10mg